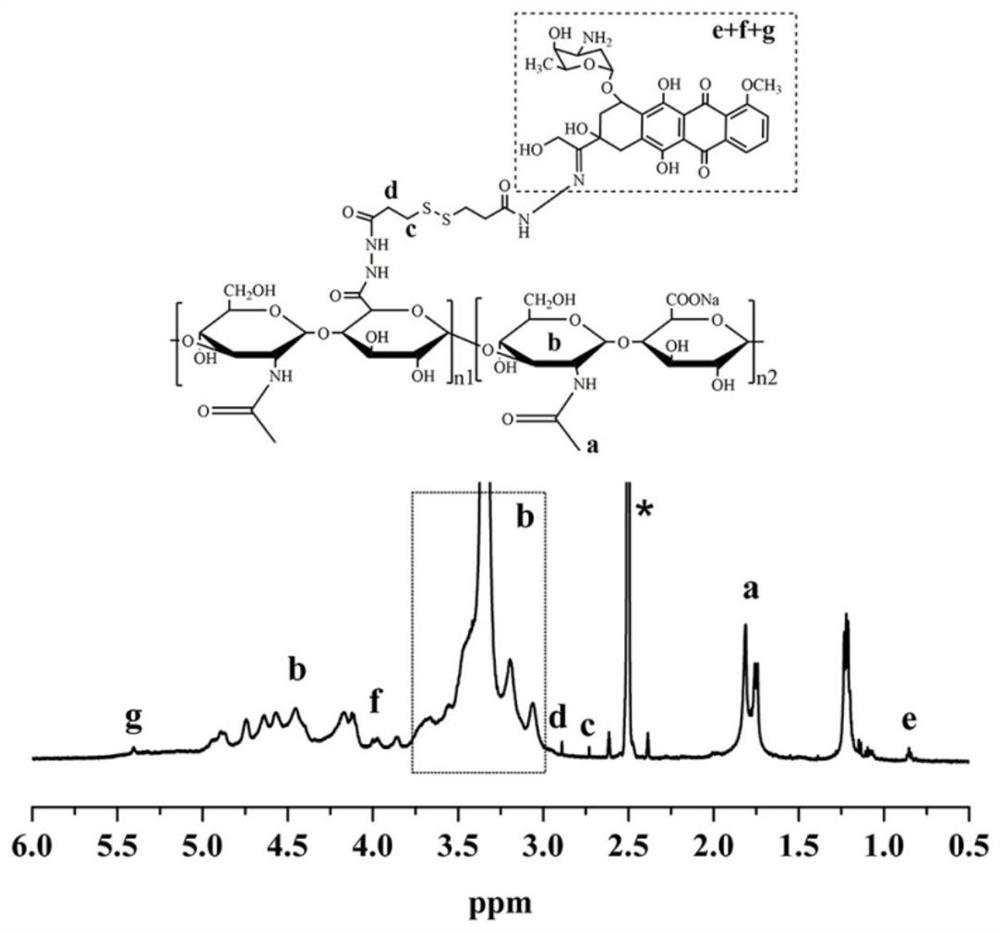
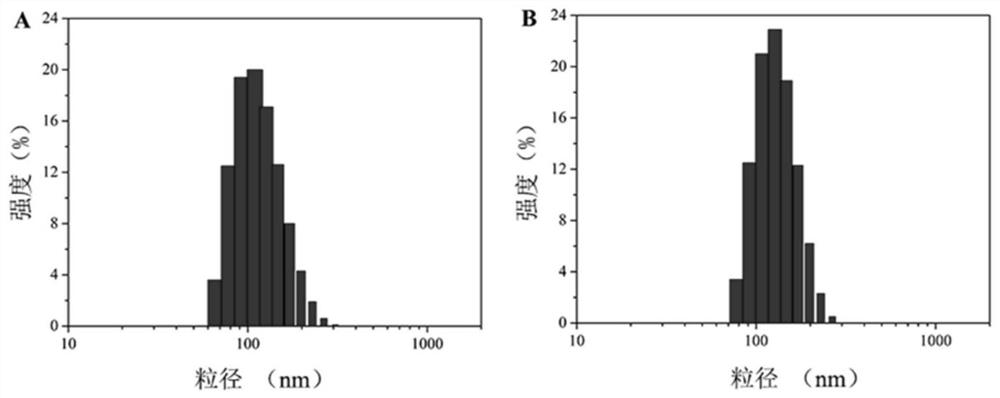
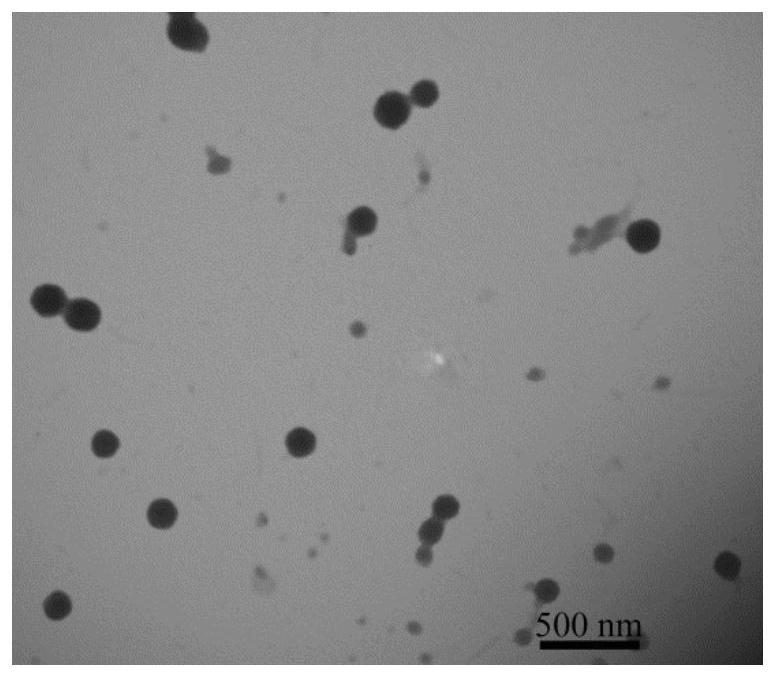
A reduction/pH-sensitive polysaccharide-based nano-prodrug co-loaded with doxorubicin and platinum drugs, its preparation method and application
A platinum-based drug, doxorubicin technology, applied in the field of reducing/pH-sensitive polysaccharide-based nano-prodrugs and its preparation, to achieve the effects of preventing leakage, increasing solubility, and rich sources of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A preparation method of reducing / pH-sensitive polysaccharide-based nano-prodrugs co-loaded with doxorubicin and platinum-based drugs. The main process is to prepare a hydrazide polysaccharide polymer by using the carbodiimide method, and then sequentially bond through hydrazone bonds. Connecting doxorubicin and coordination bonds to connect platinum drugs to obtain a reduced / pH-sensitive polysaccharide-based nano-prodrug that co-loads doxorubicin and platinum drugs. Specifically include the following steps:
[0033] (1) Preparation of hydrazide polysaccharide polymer: dissolving the carboxyl polysaccharide polymer in water to make a carboxyl polysaccharide polymer aqueous solution with a mass concentration of 0.1% to 10%, and then adding 5% to 60% of the carboxyl substance 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide, in which 1-(3-dimethylaminopropyl)-3-ethane The molar ratio of carbodiimide hydrochloride and N-hydroxysuccinimid...
Embodiment 1
[0047] (1) Preparation of hyaluronic acid hydrazide: the molecular weight is 8kDa hyaluronic acid dissolved in water to make a mass concentration of 1.0% hyaluronic acid aqueous solution, adding 1-( 3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide salt The molar ratio of acid salt and N-hydroxysuccinimide is 1:1.5, adjust the pH value to 5.3 with dilute hydrochloric acid (1mol / L) and maintain it at 5.3, add 3,3' after stirring at room temperature for 1 hour -dithiodipropionyl hydrazide, after reacting for 36 hours, the reactant was placed in a dialysis bag with a molecular weight cut-off of 3500Da and dialyzed for 48 hours, and freeze-dried at minus 20 degrees Celsius to obtain hydrazide hyaluronic acid with a hydrazide degree of 40%; wherein , The molar ratio of 3,3'-dithiodipropionyl hydrazide to 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is 5:1.
[0048] (2) Formation of doxoru...
Embodiment 2
[0058] (1) Preparation of hydrazidized hyaluronic acid: dissolve hyaluronic acid with a molecular weight of 8 kDa in water to make a hyaluronic acid aqueous solution with a mass concentration of 1.0%, add 1- (3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide The molar ratio of hydrochloride and N-hydroxysuccinimide is 1:1.5. Use dilute hydrochloric acid (1mol / L) to adjust the pH value to 5.0 and maintain it at 5.0. After stirring at room temperature for 0.5 hours, add 3,3 '-dithiodipropionyl hydrazide, after 48 hours of reaction, the reactant was placed in a dialysis bag with a molecular weight cut-off of 3500Da and dialyzed for 72 hours, and freeze-dried at minus 20 degrees Celsius to obtain hydrazide hyaluronic acid with a hydrazide degree of 20%; Wherein, the molar ratio of 3,3'-dithiodipropionyl hydrazide to 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride is 25:1.
[0059] (2) Forma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com