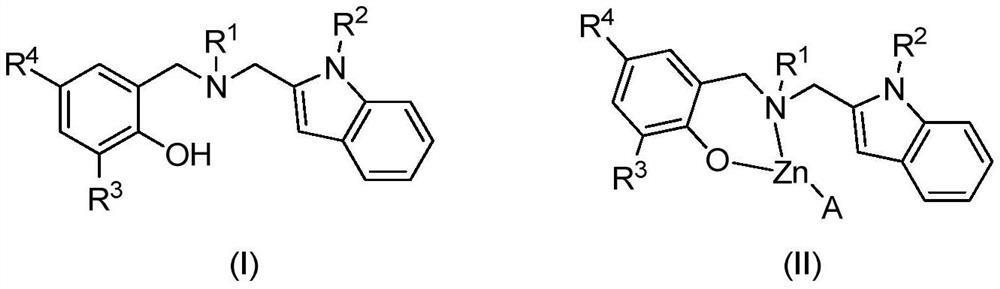
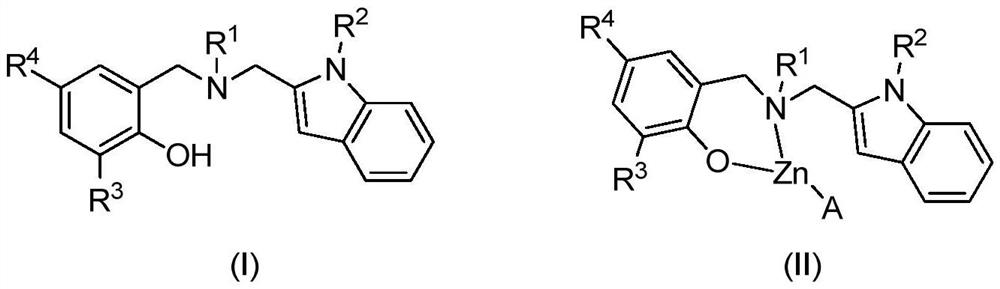
Indole ring substituted aminophenoxy zinc complex as well as preparation method and application thereof
A technology of aminophenoloxyzinc and indole ring, applied in the direction of zinc organic compounds and the like, can solve the problems of reduced catalytic activity and the like, and achieve the effects of high catalytic activity, convenient preparation and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
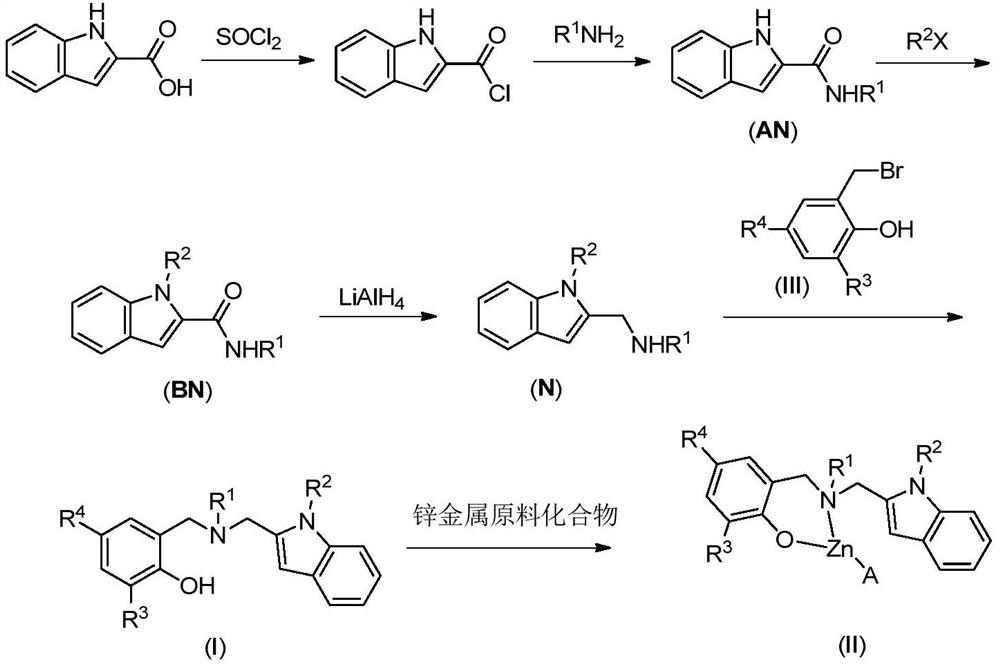
[0045] Synthesis of raw materials and intermediates:
[0046] (1) Synthesis of 2-indolecarboxamide (AN)
[0047]
[0048] Under argon protection, add 150 mL of dry CH to a 250 mL three-neck flask 2 Cl 2 , 2-indolecarboxylic acid (30mmol, 4.8g) was added and stirring was started. Then add SOCl 2 (60.0mmol, 8.57g), after heating to reflux for 2h, the solvent and excess thionyl chloride were evaporated with a rotary evaporator. Add 100mL CH 2 Cl 2 It was dissolved again, and the solvent was evaporated again with a rotary evaporator. After repeated three times, it was directly used in the next reaction.
[0049] Under argon protection, into a 250mL three-neck flask, add triethylamine (36mmol, 2.62g) and primary amine (36mmol) and 100mL dry CH 2 Cl 2 well mixed. Dissolve the 2-indolecarbonyl chloride obtained above in 50 mL of dry CH 2 Cl 2 , added dropwise to the reaction system. When preparing AN1 and AN2, a large amount of solids existed in the reaction flask afte...
Embodiment 2
[0062] Synthesis of Ligand L1
[0063] At room temperature, secondary amine N1 (3.64g, 14.2mmol), potassium carbonate (2.35g, 17.0mmol) and 40mL N,N-dimethylformamide were added to a 100mL single-necked bottle, stirred for 5min, and then added in 2 batches -Bromomethyl-4,6-dicumylphenol (5.99 g, 14.2 mmol). Continue to stir the reaction for 4h, TLC tracking shows that the reaction is complete, add 60mL water to quench, and use 30mL×3 CH 2 Cl 2 Extract, combine the organic phases, and wash with 60 mL×6 saturated brine. The organic phase was dried with anhydrous magnesium sulfate, filtered, and the filtrate was evaporated to remove the solvent with a rotary evaporator. Recrystallization from dichloromethane-petroleum ether system gave white solid product L1 (5.85 g, 41.2%).
[0064]
[0065] 1 H NMR (CDCl 3 ,400MHz,298K):δ10.06(br s,1H,OH),7.53(d, 3 J=7.8Hz, 1H, Indolyl-H), 7.33-7.11(m, 13H, 10H of ArH and 3H of Indolyl-H), 7.08(t, 3 J=7.0Hz, 1H, Indolyl-H), 6.78(d, 1...
Embodiment 3
[0067] Synthesis of Ligand L2
[0068] Reaction products were secondary amine N1 (2.69g, 10.5mmol), potassium carbonate (1.74g, 12.60mmol) and 2-bromomethyl-4-methyl-6-tritylphenol (4.65g, 10.5mmol) In addition, other operations were consistent with the synthesis of L1, and recrystallized from dichloromethane-petroleum ether system to obtain white solid L2 (3.93 g, 60.5%).
[0069]
[0070] 1 H NMR (CDCl 3 ,400MHz,298K):δ10.38(br s,1H,OH),7.53(d, 3 J=7.7Hz, 1H, Indolyl-H), 7.25-7.05(m, 18H, 15H of ArH and 3H of Indolyl-H), 6.88(d, 1H, 4 J=1.6Hz, ArH), 6.76(d, 1H, 4 J=1.6Hz, ArH), 6.22(s, 1H, Indolyl-H), 3.84-3.77(s, 2H, ArCH 2 N; q, 3 J=7.0Hz, 2H, NCH 2 CH 3 ),3.68(s,2H,Indolyl-CH 2 N),2.47(pesudo t,1H, 3 J=7.0Hz, NCH), 2.16(s, 3H, ArCH 3 ),1.80-1.65(m,4H,CH 2 of cyclohexyl),1.63-1.49(m,2H,CH 2 of cyclohexyl),1.37-1.23(m,2H,CH 2 of cyclohexyl),1.02-1.15(m,5H,2H ofcyclohexyl and 3H of NCH 2 CH 3 ). 13 C{ 1 H}NMR (CDCl 3 , 100MHz, 298K): δ154.2, 146.2, 13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com