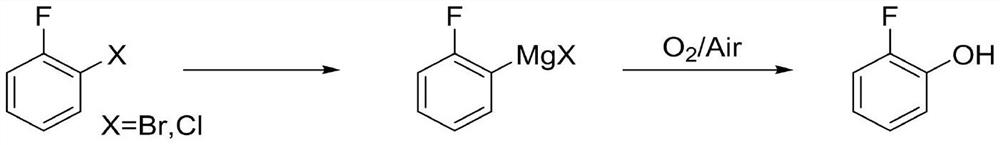
Method for preparing o-fluorophenol by one-pot method
A technology of o-fluorophenol and chlorofluorobenzene, which is applied in the field of preparation of o-fluorophenol, can solve the problems of strong toxicity of fluorine-containing reagents, unstable diazotization, and explosives, and avoid fluorinated reagents and unstable diazonium Chemical reaction, process efficiency, no isomer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
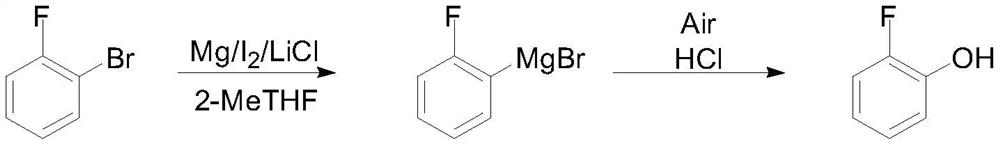
[0023] Under the protection of nitrogen flow, put 13g (0.542mol) of magnesium metal, 8.6g (0.049mol) of o-bromofluorobenzene, 31.2g (0.736mol) of anhydrous lithium chloride and 150mL of 2-methyltetrahydrofuran into the reaction flask, and cool down to - 10°C, add 0.1g iodine and 1g dibromoethane to initiate, after the initiation, slowly add dropwise the remaining 77.4g o-bromofluorobenzene and 300mL 2-methyltetrahydrofuran mixed solution, after dropping, slowly raise the temperature to 25°C for reaction 2 Hours, sampling dilute hydrochloric acid quenched and organic solvent extraction, detection of raw materials without remaining, containing 3% benzene. Cool down to 0°C, control the flow rate and feed compressed air, keep it warm for 6 hours, slowly raise the temperature to 35°C and keep it warm for 2 hours, take a sample to detect the product purity is greater than 96%, cool down to 10°C, add dilute hydrochloric acid to quench, separate, and water phase Extract wi...
Embodiment 2
[0025]
[0026] Under the protection of nitrogen flow, put 65.3g (0.5mol) of o-bromofluorobenzene, 27.5g (0.736mol) of anhydrous lithium chloride and 180mL of tetrahydrofuran into the reaction bottle, lower the temperature and control it at -5°C, and slowly add 262mL of isopropyl Magnesium chloride / tetrahydrofuran solution (2.0mol / L), reacted at this temperature for 3 hours, raised the temperature to 15°C and reacted for 2 hours, sampled dilute hydrochloric acid to quench and extracted with organic solvent, no raw materials were detected, containing 1.5% benzene. Cool down to 0°C, control the flow rate and feed compressed air, keep it warm for 6 hours, slowly raise the temperature to 35°C and keep it warm for 2 hours, take a sample to test that the product purity is greater than 93%, cool down to 10°C, add acetic acid aqueous solution to quench, separate layers, and water phase Extract with ethyl acetate, combine the organic phases, add activated carbon and sodium thiosulfat...
Embodiment 3
[0028]
[0029] Under the protection of nitrogen flow, put 65.3g (0.5mol) of o-bromofluorobenzene, 27.5g (0.736mol) of anhydrous lithium chloride and 180mL of tetrahydrofuran into the reaction bottle, lower the temperature and control it at -5°C, and slowly add 261mL of ethylmagnesium chloride dropwise Tetrahydrofuran solution (2.0 mol / L), reacted at this temperature for 2 hours, raised the temperature to 15°C and reacted for 2 hours, sampled dilute hydrochloric acid to quench and extracted with organic solvent, no remaining raw materials were detected, containing 1.8% benzene. Cool down to 0°C, control the flow rate to feed oxygen, keep warm for 2 hours, slowly raise the temperature to 35°C and keep warm for 3 hours, take a sample to test the product purity is 92%, cool down to 10°C, add ammonium chloride aqueous solution to quench, separate layers, water phase acetic acid Extract with ethyl ester, combine the organic phases, add activated carbon and sodium thiosulfate, fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com