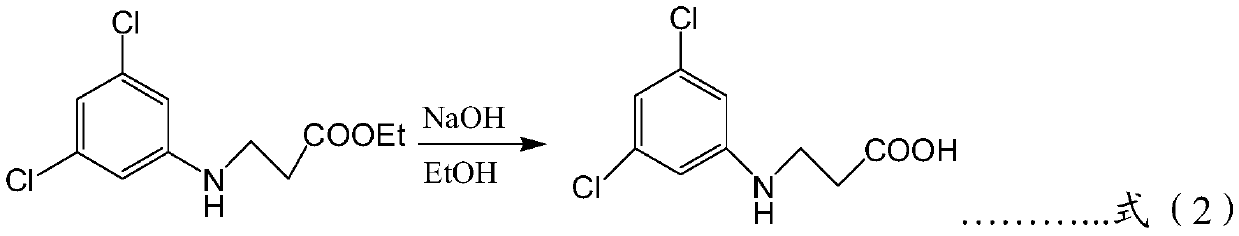
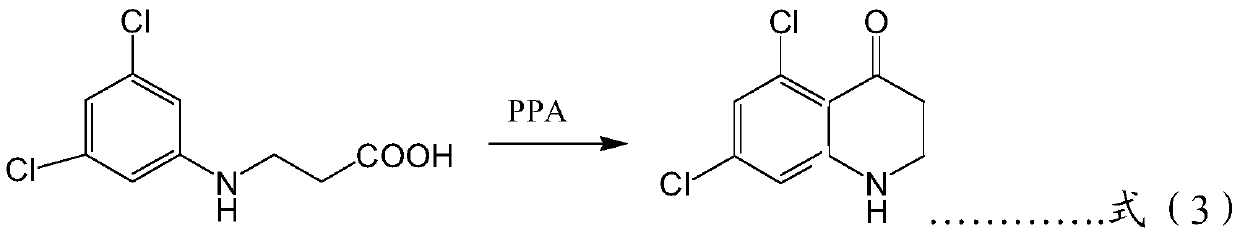
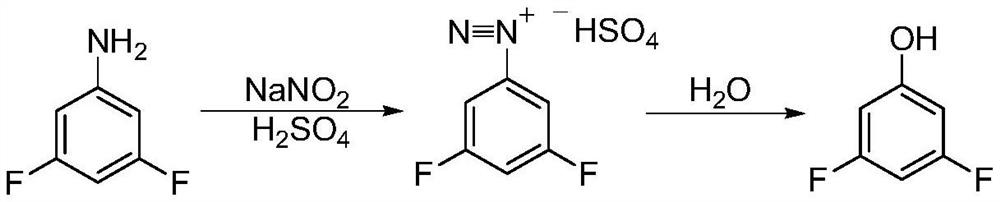
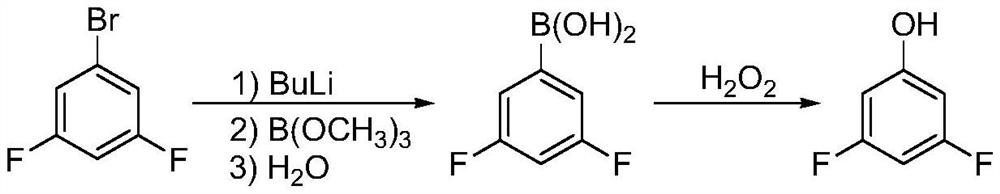
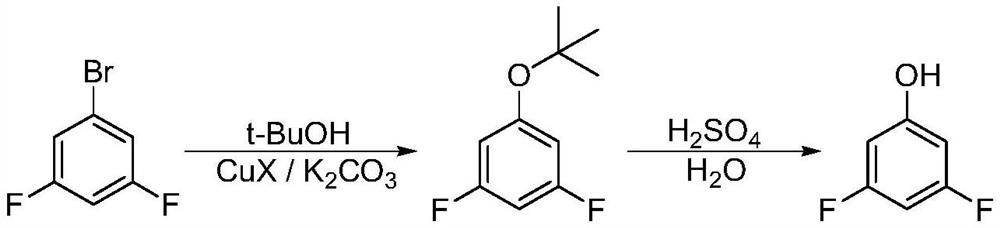
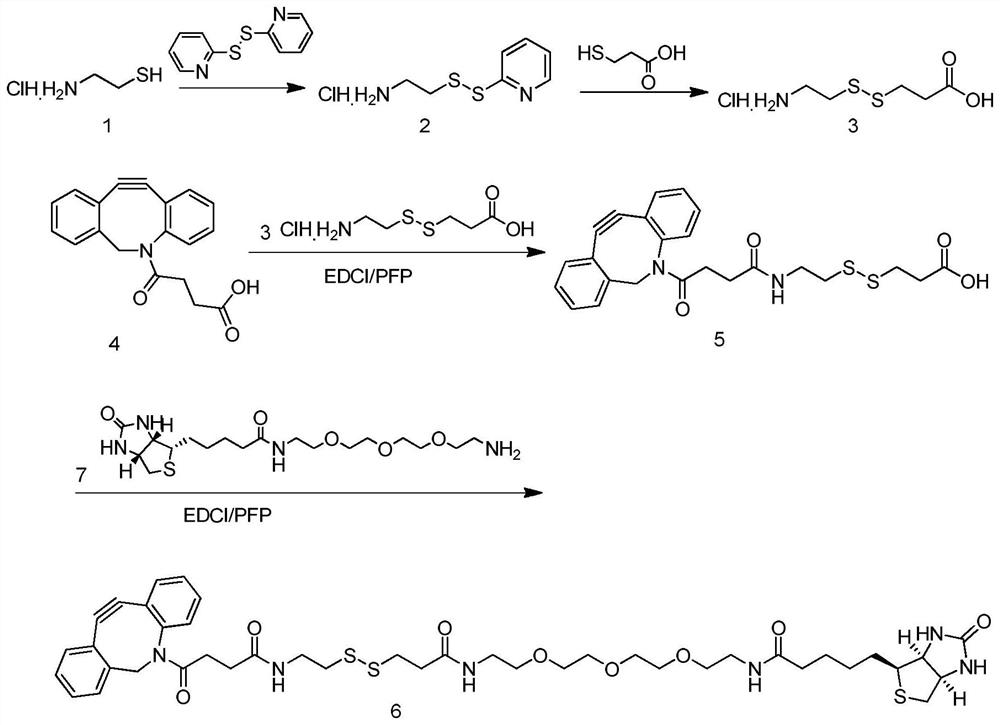
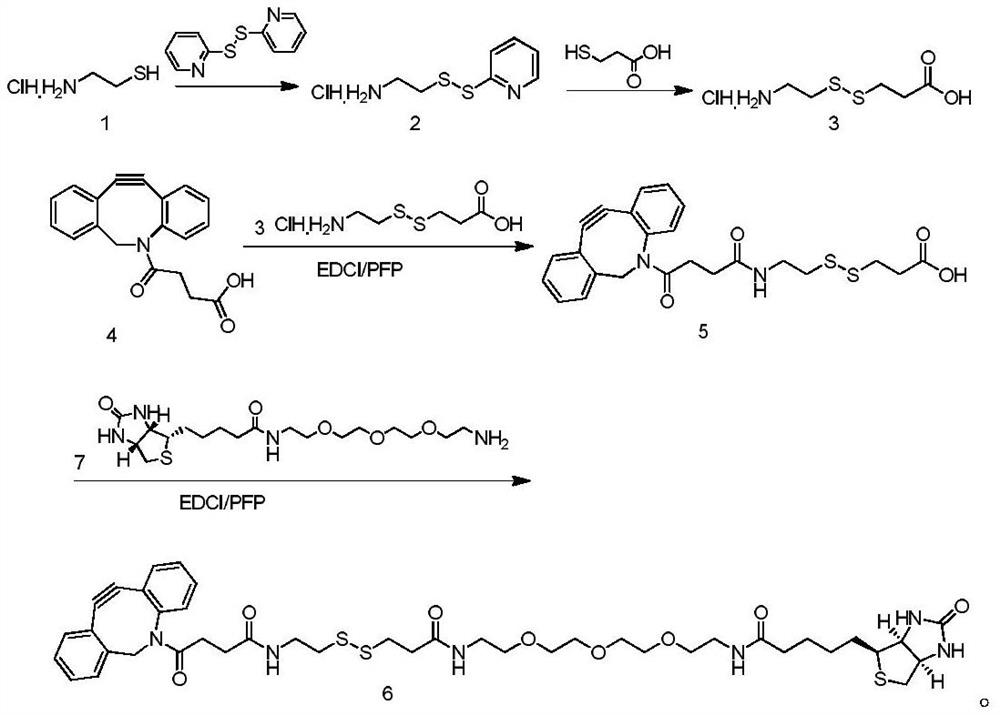
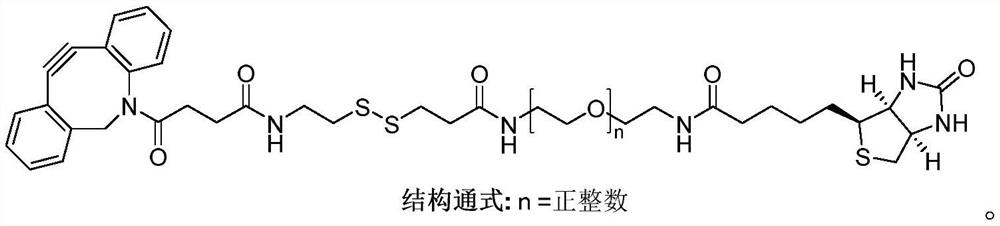
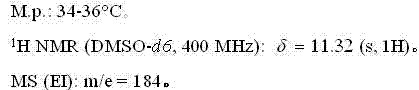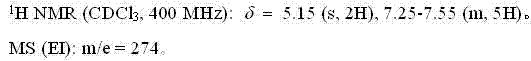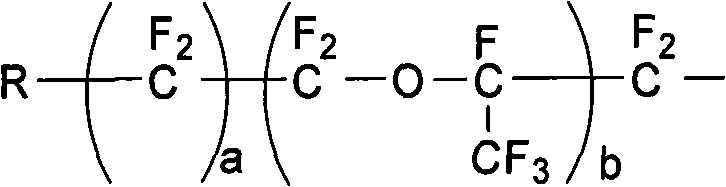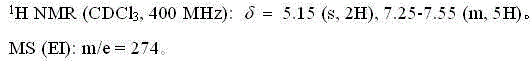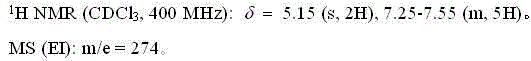Patents
Literature
44 results about "Fluroxene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
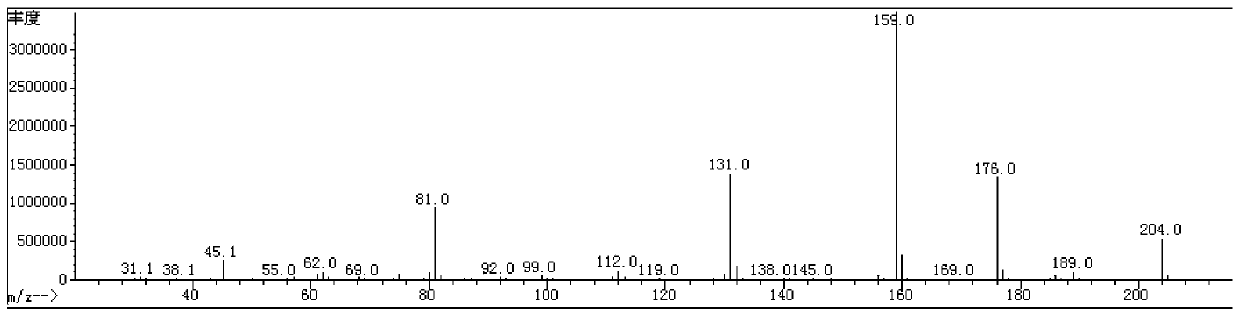
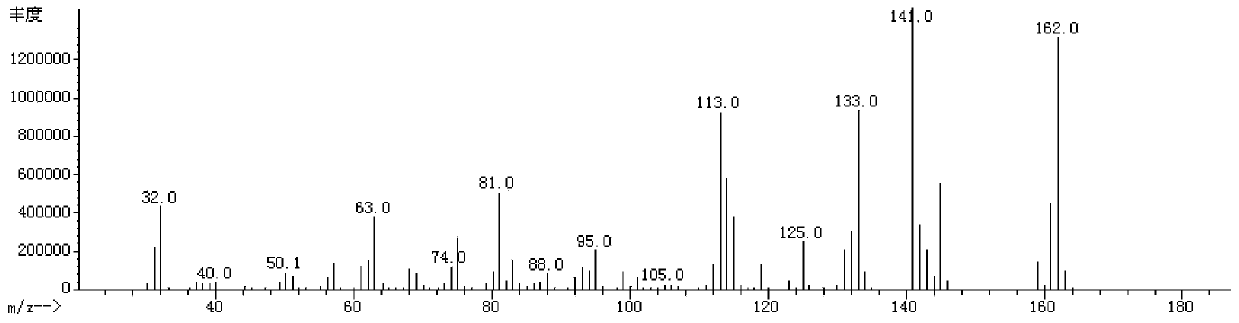
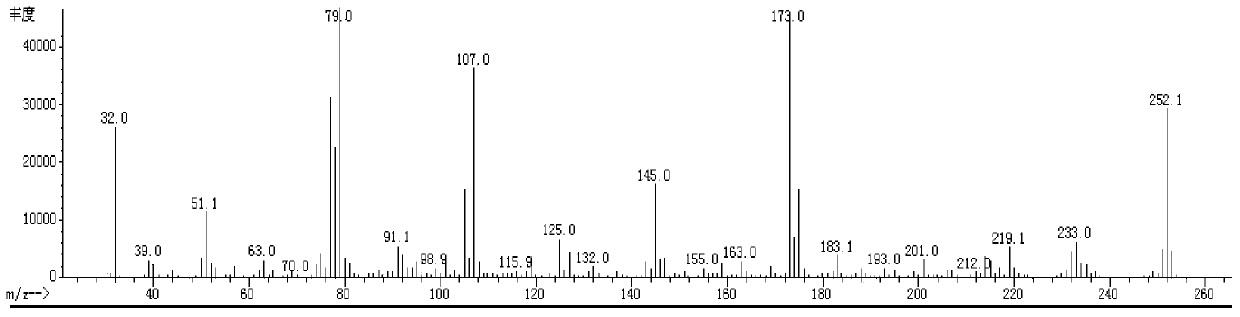
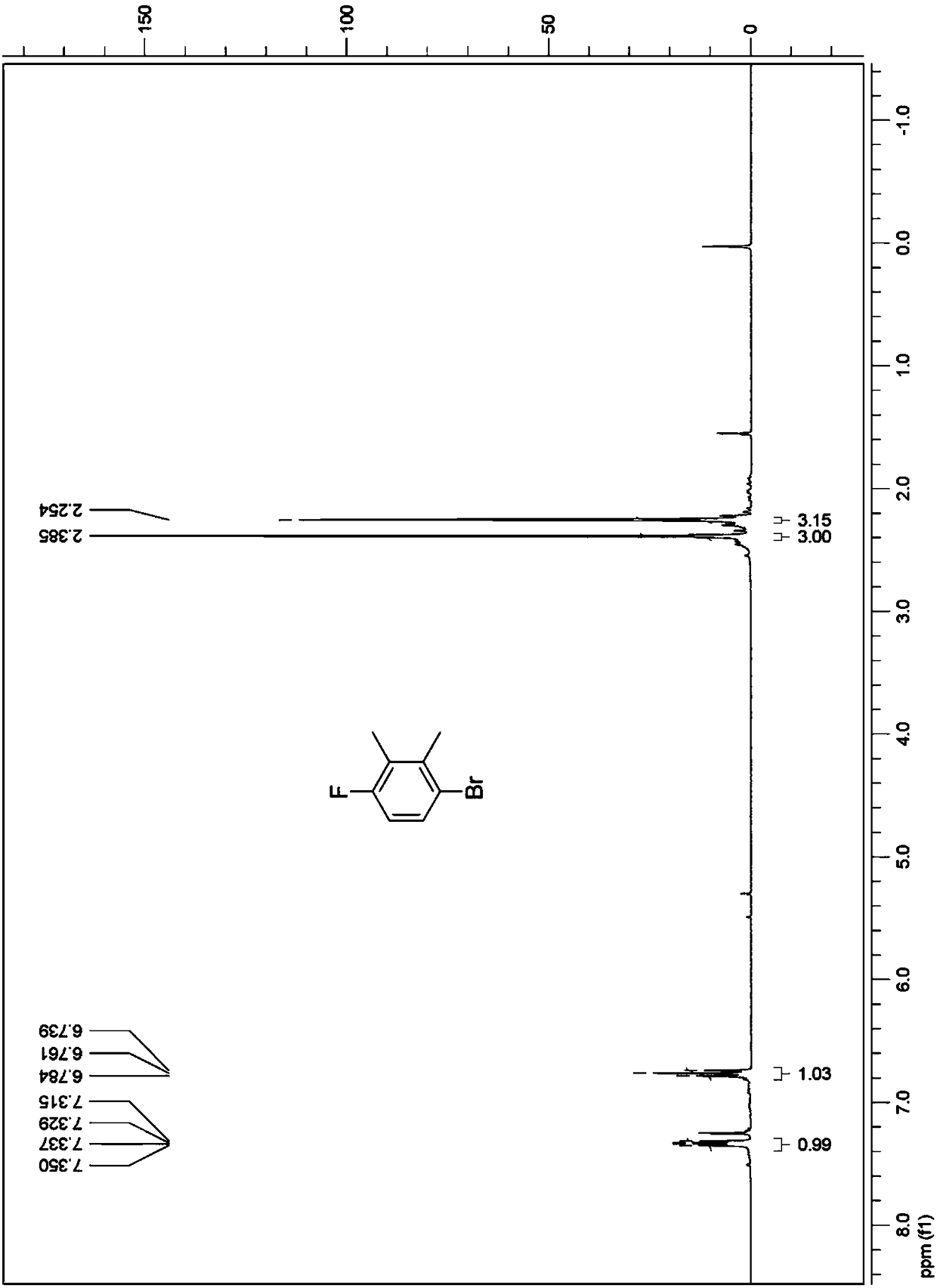
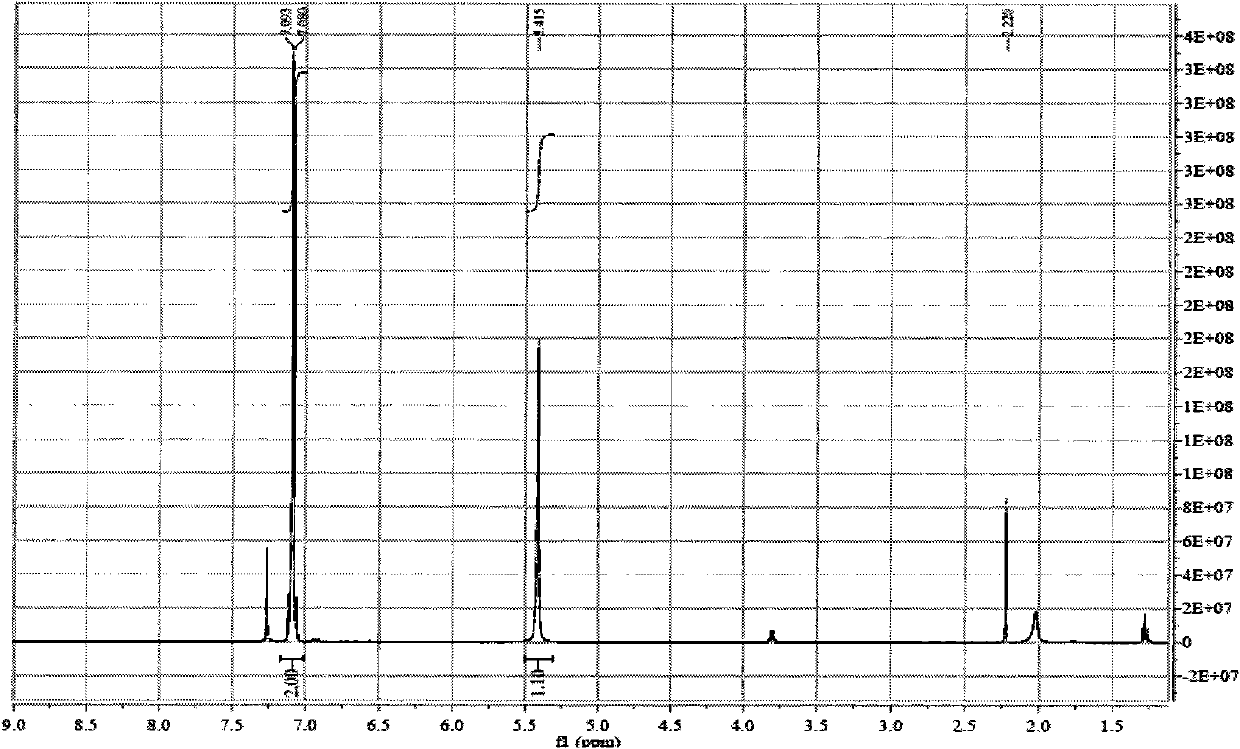
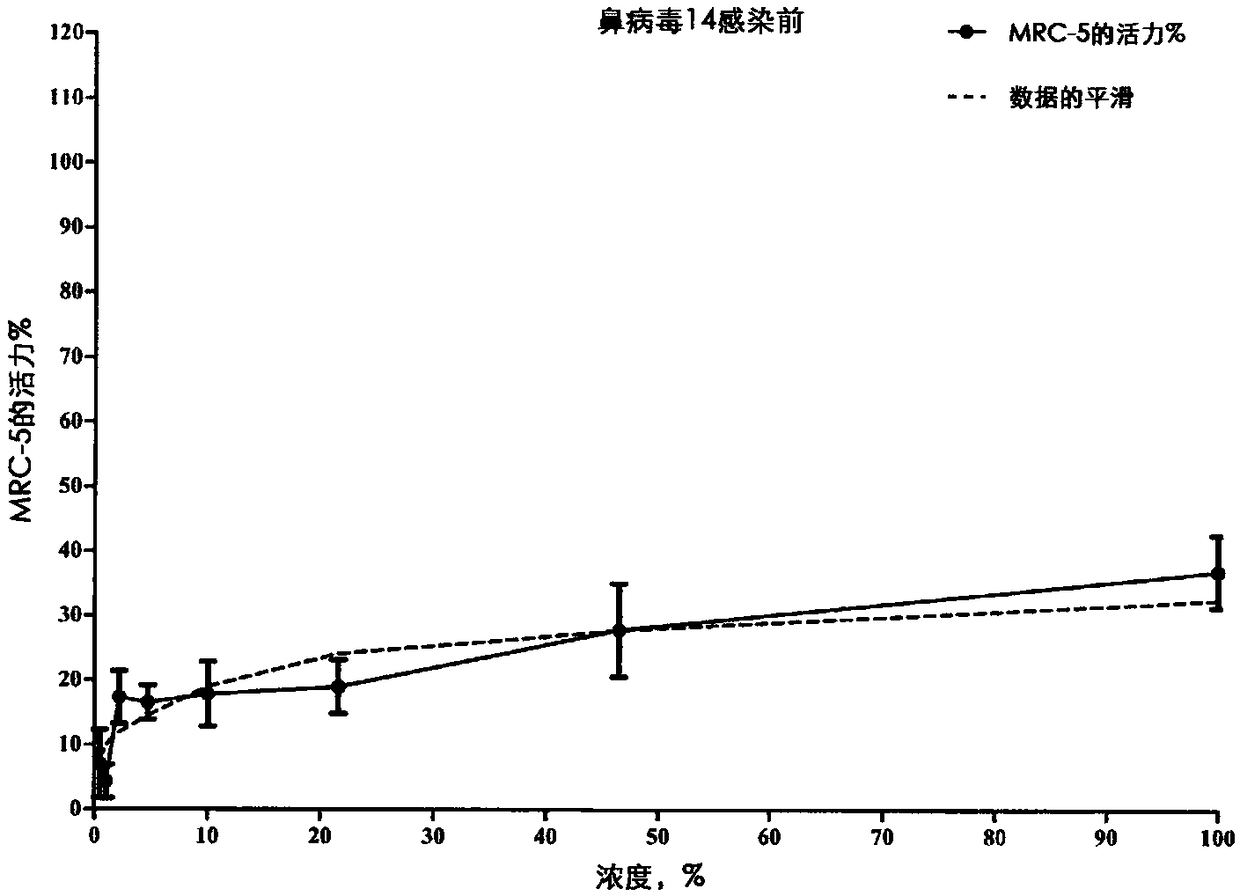
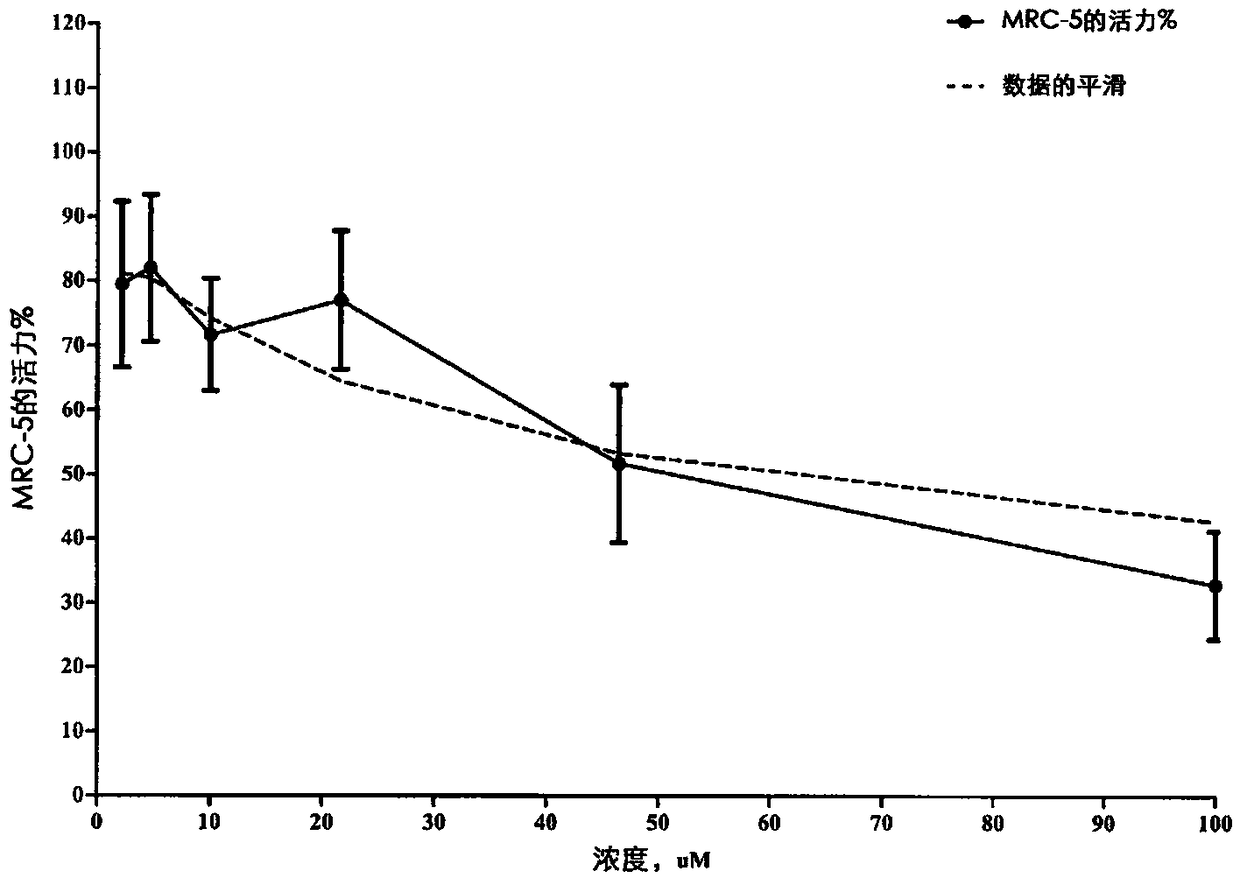
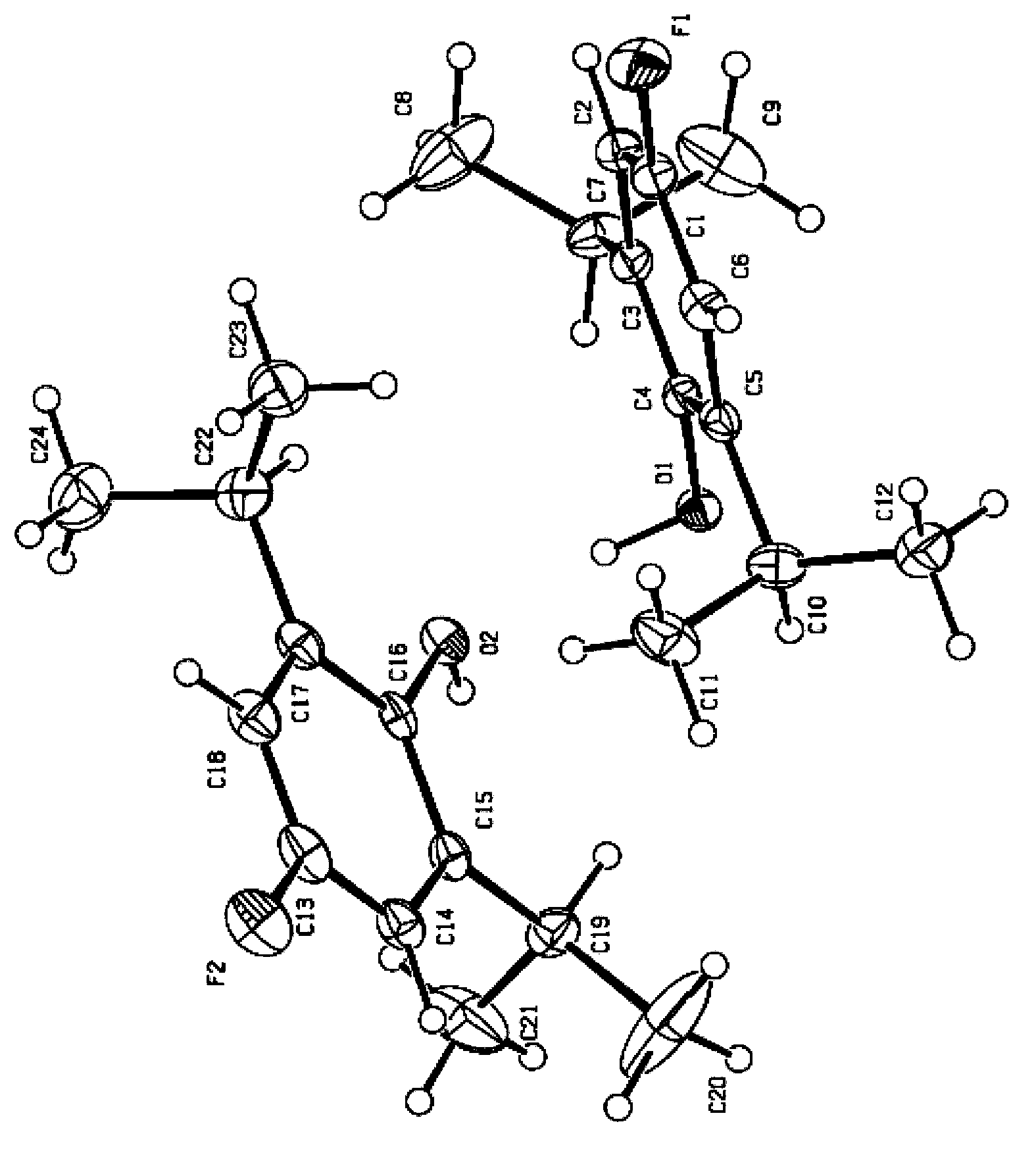
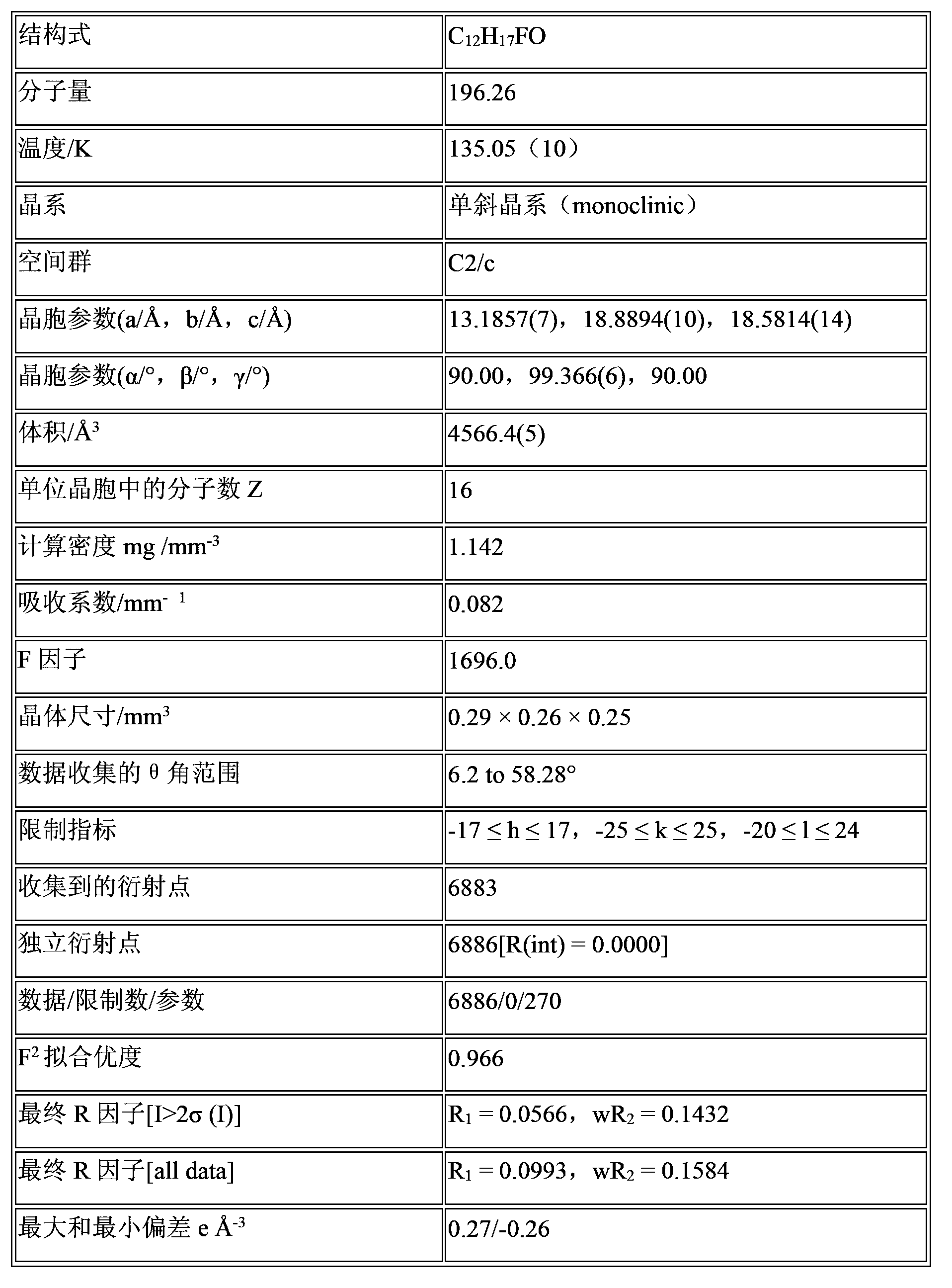
Fluroxene (INN, USAN; brand name Fluoromar), or 2,2,2-trifluoroethyl vinyl ether, is a volatile, inhalational anesthetic, and was the first halogenated hydrocarbon anesthetic to be introduced. It was synthesized in 1951, and was introduced for clinical use in 1954, but was voluntarily withdrawn from the market in 1974 due to its potential flammability and accumulating evidence that it could cause organ toxicity. In any case, prior to being discontinued, it had largely been superseded by halothane. Fluroxene is metabolized to 2,2,2-trifluoroethanol, a compound responsible for some of the toxicity seen with fluroxene use.
Cotton fibre and blended fabric printing and dyeing method
The invention relates to the dye printing technology of cotton fiber and blend fabric, in particular to a dye printing processing method of cotton fiber and blend fabric. A double blade coating machine is adopted, the dyeing and the printing are simultaneously realized on one machine; invention comprises the following steps: firstly, the pad dyeing is performed to the textile in colored or colorless padding dye liquor, the padding liquor comprises the following components according to the weight percentage: penetrating agent organosilicon fluroxene poly occupies 0 to 2 percent, branched chain aliphatic condensed alcohol ethylene oxide occupies 1 to 2 percent, softening agent organosilicon occupies 0 to 2 percent, modified 2D resin occupies 0 to 8 percent, water is quantified to 100 percent; secondly, the single side dyeing and drying of the textile is performed through a first blade, thirdly, color pastes which have more than two colors and can not be mixed and dissolved are scraped to the textile and the textile is dried. The invention cause two styles of synthesis dyeing and printing of textile can be completed on single coating equipment, the process is simple, and no waste water is discharged.
Owner:DANDONG UNIK TEXTILE
2,3,5-Trifluoro-4-difluoro(3,4,5-trifluorophenylol)methyl-benzaldehyde, its synthetic method and its application in preparation of liquid crystal compound
ActiveCN104513145ALow cost of purchaseGood yieldLiquid crystal compositionsOrganic compound preparationBenzaldehydeLiquid crystal
The invention provides 2,3,5-trifluoro-4-difluoro(3,4,5-trifluorophenylol)methyl-benzaldehyde (a compound of formula 8), and its preparation method. The compound can be used to synthesize a compound with the structure represented by formula I as an intermediate compound. The compound of formula 8 is prepared from 2,3,4,5-tetrafluorobenzoic aid. The method has the advantages of simple operation, high yield, low cost, and suitableness for industrial production.
Owner:JIANGSU HECHENG ADVANCED MATERIALS
Method for synthesizing 2, 3-dimethyl-4-fluorophenol
ActiveCN108069831AFew reaction stepsHigh selectivityOrganic compound preparationHalogenated hydrocarbon preparationN dimethylformamideEconomic benefits
The invention discloses a method for synthesizing 2,3-dimethyl-4-fluorophenol. The method comprises the following steps: adding aluminum trichloride in 2,3-dimethylfluorobenzene at a normal temperature and carrying out bromination reaction to obtain 3-bromo-6-dimethylbenzene; carrying out methoxylation on the 3-bromo-6-dimethylbenzene under the effect of N,N-dimethylformamide and sodium methoxideto obtain 3-methoxyl-6-dimethylbenzene; and carrying out hydrolysis on the 3-methoxyl-6-dimethylbenzene to obtain 2,3-dimethyl-4-fluorophenol. According to the synthesizing method, the 2,3-dimethylfluorobenzene which is low in price is used as a reaction raw material, in every step of reaction, an intermediate which is high in purity can be obtained to a maximum extent, thus, the total yield of total reaction is high, the purity of the final product reach up to 98% or above, the economic benefit is quite obvious, and the method is particularly suitable for industrial large-scale application and popularization.
Owner:SHANGHAI SINOFLUORO SCI
Synthesis method of 1-cyclopropyl-4-oxo-7-fluoro-8-methoxy-1,4-dihydroquinolyl-3-carboxylic acid
ActiveCN103819401AStable market supplyObvious cost advantageOrganic chemistryOrganolithium reagentEther
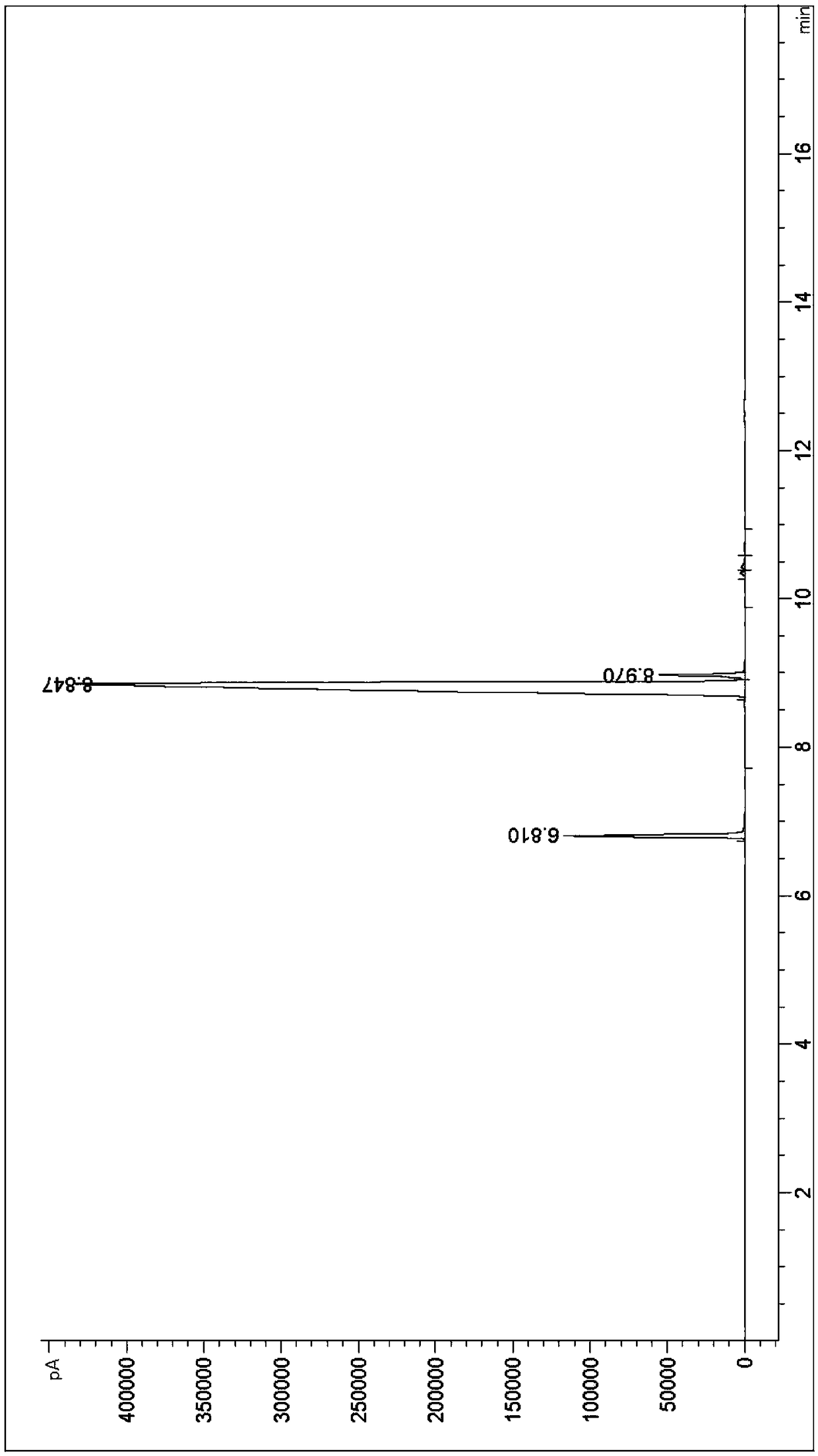
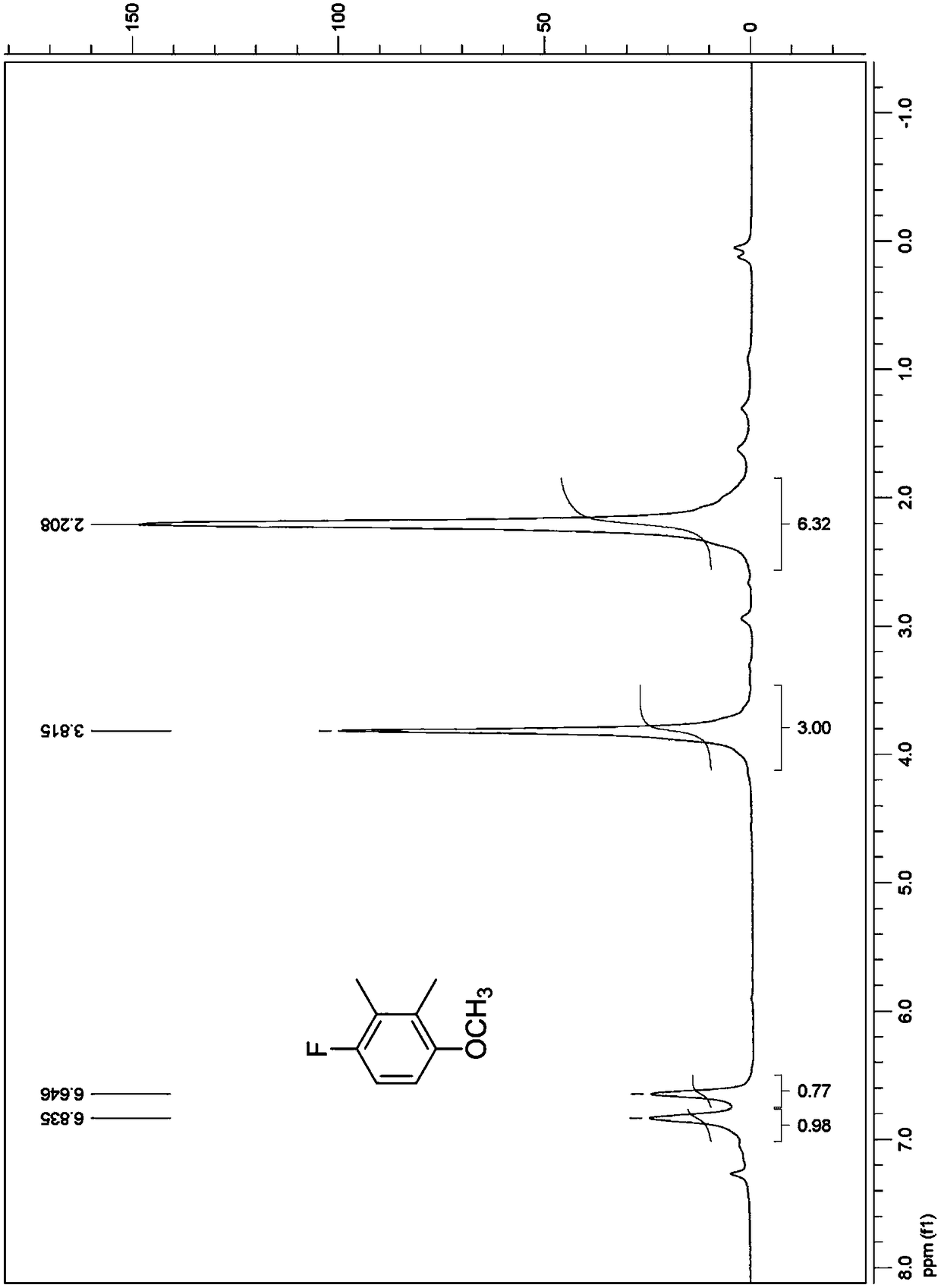
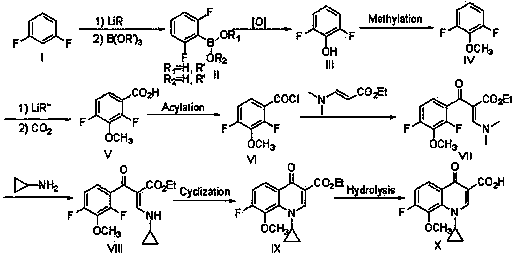
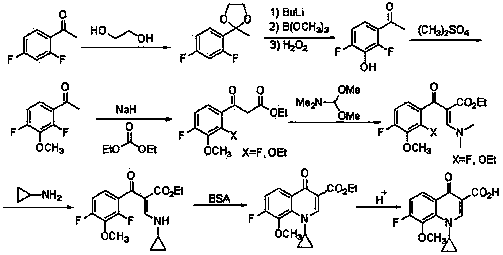
The invention discloses a synthesis method of 1-cyclopropyl-4-oxo-7-fluoro-8-methoxy-1,4-dihydroquinolyl-3-carboxylic acid. The synthesis method comprises the following reaction steps: reacting m-difluorophenyl with an organolithium reagent, reacting an obtained aryllithium intermediate with borate, quenching to obtain 2,6-difluorophenylboronic acid and 2,6-difluorophenylborate, reacting 2,6-difluorophenol obtained through oxidation with a methylating reagent, reacting the obtained 2,6-difluorophenylmethyl ether with the organolithium reagent, reacting the obtained corresponding aryllithium intermediate with carbon dioxide, reacting the obtained product with an acylchlorinating reagent to obtain 2,4-difluoro-3-methoxy benzoyl chloride, and performing cyclization, hydrolysis and the like to obtain the 1-cyclopropyl-4-oxo-7-fluoro-8-methoxy-1,4-dihydroquinolyl-3-carboxylic acid. The synthesis method has the advantages as follows: raw materials are easy to obtain; the yield in each step is high; the atom economy is high; the synthesis method is suitable for industrial application.
Owner:ZHEJIANG ZHONGXIN FLUORIDE MATERIALS CO LTD
Method for synthesizing 2,3,4,5,6-pentafluorophenol
ActiveCN103787839AReduce usageLow costOrganic compound preparationEther preparation by ester reactionsPalladium on carbonPtru catalyst
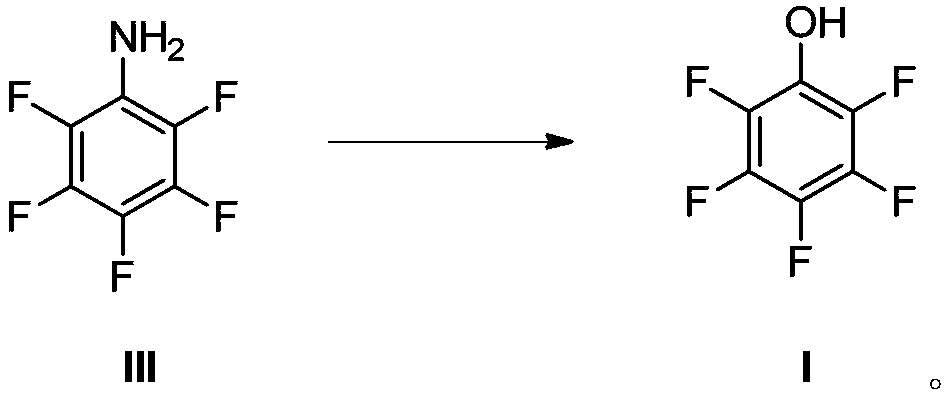
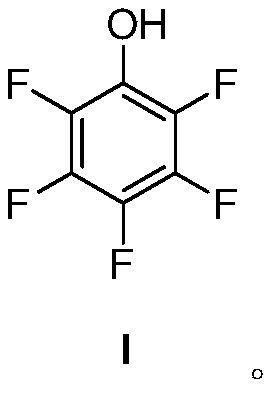
The invention provides a method for synthesizing 2,3,4,5,6-pentafluorophenol. The method comprises the steps of (a) carrying out Ullmann reaction on bromopentafluorobenzene as shown in a formula (I) and benzyl alcohol in an inert gas atmosphere under the action of a catalyst cuprous iodide, a ligand and an inorganic base at 100-120 DEG C, and then generating a compound (II), and (b) reacting the compound (II) in an alcoholic solvent in the presence of hydrogen under the action of a palladium carbon catalyst at 20-50 DEG C, and removing the benzyl group, thereby obtaining a compound (III), namely, the 2,3,4,5,6-pentafluorophenol. The method is simple, low in cost and high in yield, and applicable to industrial production. The synthetic route of the method is as shown in specification.
Owner:SUZHOU HIGHFINE BIOTECH
Preparation method of 3,5-difluoro-4-trifluoro-methoxyl bromobenzene
The invention relates to the field of a liquid-crystal intermediate of an electronic material, in particular to a preparation method of a fluorine-containing liquid-crystal intermediate 3, 5-difluoro-4-trifluoro-methoxyl bromobenzene. The 3,5-difluoro-4-trifluoro-methoxyl bromobenzene is prepared from 2,6-difluoro phenol by bromine substitution and coupling. The preparation method of the 3,5-difluoro-4-trifluoro-methoxyl bromobenzene has the advantages of mild reaction condition, strong operability, high atom economy, simple process and high-purity and stable-quality obtained product and is beneficial to the realization of industrialization, has and completely accords with the use requirement as the liquid-crystal intermediate.
Owner:ZHEJIANG YONGTAI TECH CO LTD
4-amino-3-fluorophenol and synthesizing method thereof
InactiveCN105801433AImprove protectionReduce volatilityOrganic compound preparationBulk chemical productionNitrationNitrogen
The invention discloses 4-amino-3-fluorophenol and a synthesizing method thereof.The synthesizing method comprises the steps that fluoroaniline serves as a raw material, a diazo-reaction is carried out first, then a fluorine resolving reaction is carried out, and a white diazonium salt solid is obtained; then, the white diazonium salt solid is subjected to a hydrolysis reaction, and 3-fluorophenol is obtained; 3-fluorophenol is then subjected to a nitration reaction, and 3-fluorine-4-nitrophenol is obtained; then, obtained 3-fluorine-4-nitrophenol is reduced, and 4-amino-3-fluorophenol is obtained.According to 4-amino-3-fluorophenol and the synthesizing method thereof, ionic liquid serves as a reaction solvent, the volatility is low, environment is easily protected, and no harm is caused to the human body; in the reaction of reducing nitro groups into amino groups, the microwave heating mode is adopted, so that the reaction efficiency is greatly improved, and the product yield is increased.
Owner:叶芳
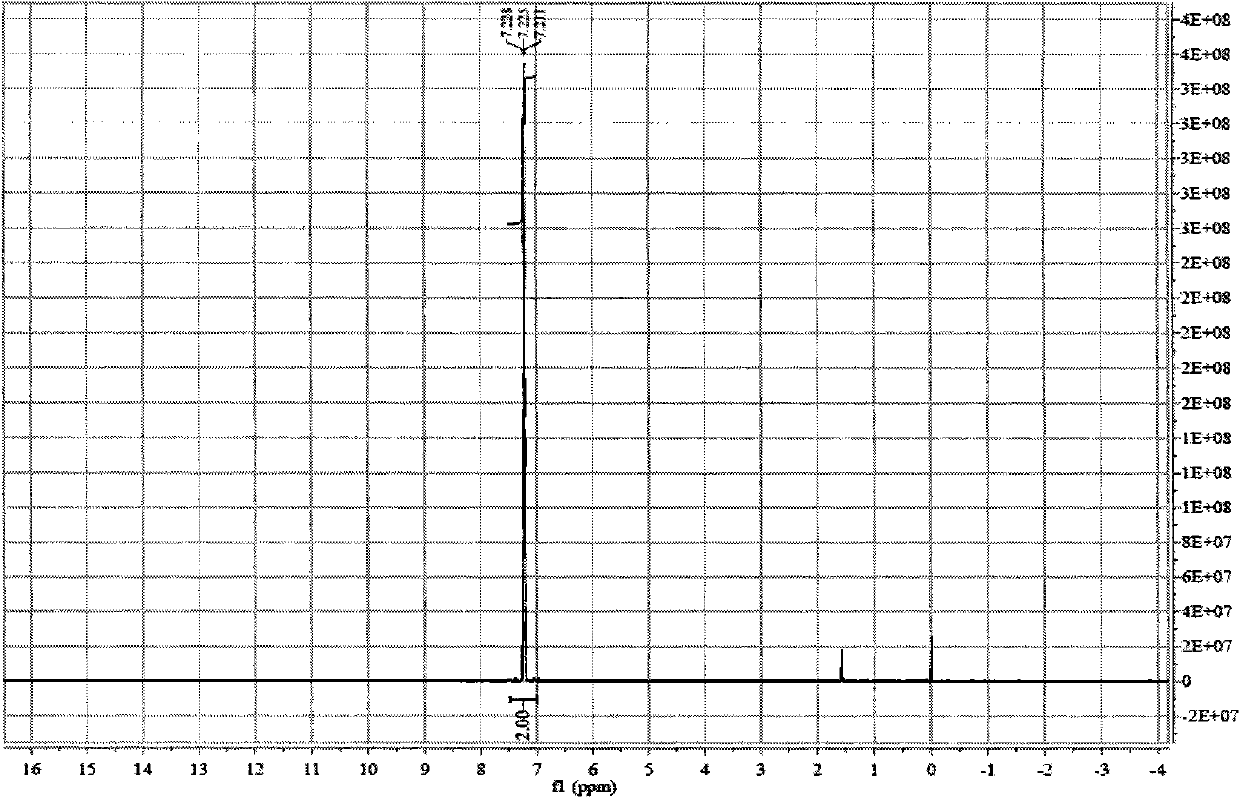
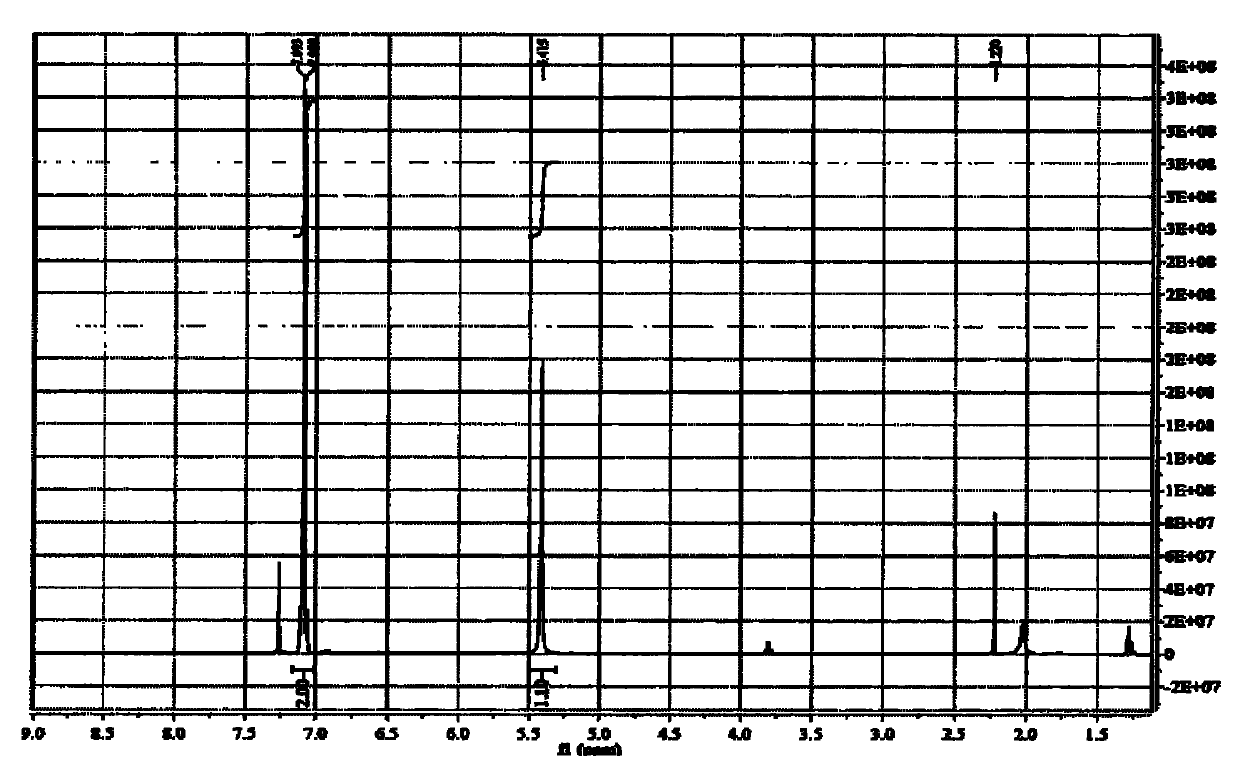
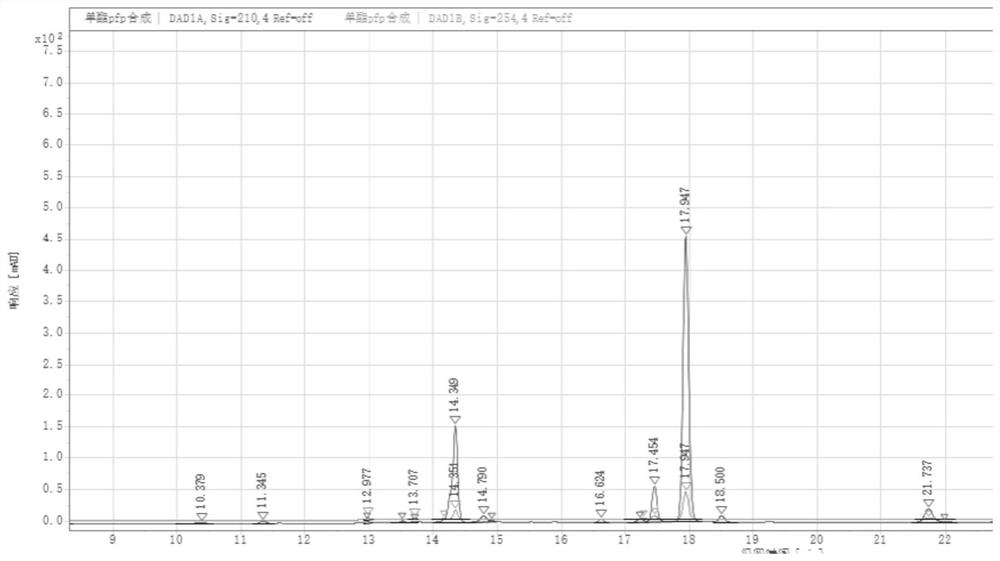
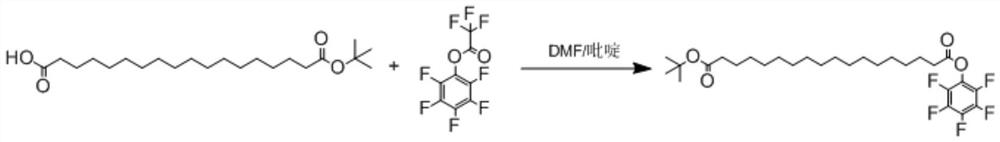
Preparation method of octadecanedioic acid mono-tert-butyl ester-PFP
PendingCN113461519ASimple reactivitySimple processing capacityOrganic compound preparationPreparation by transesterificationTrifluoroacetic acidSide chain
The invention discloses a preparation method of octadecanedioic acid mono-tert-butyl ester-PFP. The preparation method comprises the steps of preparation of a compound 1 and preparation of octadecanedioic acid mono-tert-butyl ester-PFP; wherein the preparation method of the octadecanedioic acid mono-tert-butyl ester-PFP comprises the following steps: A1, adding a compound 1, pyridine and DMF into a reaction kettle, starting stirring for completely dissolving the components, dropwise adding trifluoroacetic acid pentafluorophenol ester, separating out a solid, and reacting the components; A2, post-treatment: carrying out suction filtration on the reaction liquid, collecting light yellow or white solids, carrying out DMF pulping and suction filtration, collecting products, and carrying out vacuum drying to obtain 1.4 Kg of products. Herein,the prepared compound 1 is simple in reaction and post-treatment and can also be directly used for preparing octadecanedioic acid mono-tert-butyl ester-PFP, and a solid generated in the reaction for preparing the octadecanedioic acid mono-tert-butyl ester-PFP is the product, and a high-purity product can be obtained only by simple pulping. The coupling difficulty of octadecanedioic acid or tert-butyl ester of octadecanedioic acid used for preparing side chains of polypeptides such as semaglutide and the like is relatively high, while the octadecanedioic acid mono-tert-butyl ester-PFP can react only by adding alkali, so that the reaction efficiency is high.
Owner:浙江泽瑞生物医药有限公司
Method for preparing fluroxene by vertical-tube type catalytic reaction
ActiveCN101712619AHigh purityIncrease profitCarboxylic acid nitrile preparationOrganic compound preparationVinyl etherVertical tube
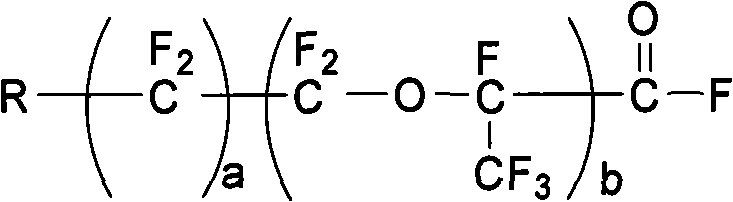
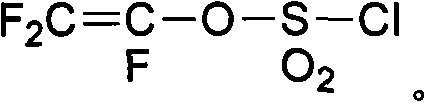
The invention discloses a preparation method of fluorine-containing vinyl ether. The method comprises the following steps of: uniformly mixing a perfluoro-linear chain or branched chain acyl fluoride compound, trifluoroethylene sulfuric ester and anhydrous hydrogen fluoride liquid in the mole ratio of 1:1:(1-20); passing from bottom to top through a vertical-tube type reactor which is filled with a catalyst; finishing addition reaction in a short time under the action of the high specific surface area catalyst inside the reactor; and setting apart a reaction liquid product from the upper part of the vertical-tube type reactor to form a product with a general formula in formula (I). In the invention, a functional fluorine-containing monomer can be prepared continuously and efficiently with no adoption of high temperature pyrolysis path, easily operated reaction equipment, conveniently controlled reaction temperature and no use of any organic solvent, and a high-purity monomer can be obtained through simple rectification. The reaction is carried out continuously inside the reactor and satisfies industrialization requirements. CF2=CF-O-Rf (I).
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Electrophilically enhanced phenolic compounds for treating inflammatory related diseases and disorders
A therapeutic compound has a modified phenolic compound of the general formula (I) wherein at least one of R, R1, R2, R3, and R4 is an electrophilic group chosen fromhalogen, aldehyde, haloalkane, alkene, butyryl, flurophenol, sulfonamide, flurophenol sulfoxide and the remaining R, R1, R2, R3, and R4 is are each independently hydrogen, a hydroxyl group, an alkoxy group, a rutinosyl group, a carboxyl group, chromone, benzopyran, a rhamnosyl group, a substituted alkoxy group or a substituted acyloxy group wherein the substituent is chosen from hydroxyl, alkoxy, aryloxy, phenyl, halogen, and amido group. The compound can be used in a therapeutic treatment of inflammatory related diseases and condition.
Owner:GLOBAL BIOLIFE INC
2,6-diisopropyl-4-fluorophenol or salt and crystal form thereof as well as preparation method and application thereof
InactiveCN103896743AHigh purityImprove biological activityOrganic active ingredientsNervous disorderSingle crystalBULK ACTIVE INGREDIENT
The invention discloses a preparation method of 2,6-diisopropyl-4-fluorophenol or a salt thereof, a crystal form of the 2,6-diisopropyl-4-fluorophenol, a medicine composition using the compound as an active ingredient, and an application thereof. The 2,6-diisopropyl-4-fluorophenol is prepared through phenolic hydroxyl group protection, ammoniation, fluorination and deprotection by using 2,6-diisopropyl-4-bromophenol as a starting raw material. The preparation method disclosed by the invention has the advantages of availability of the raw material, low cost, and simplicity in operation; and the 2,6-diisopropyl-4-fluorophenol is easily purified, a single crystal with high purity and the crystal form suitable for officinal application can be obtained. The 2,6-diisopropyl-4-fluorophenol or salt thereof has a valuable pharmacological property, and can be applied to preparation of central nervous system drugs.
Owner:SICHUAN HAISCO PHARMA CO LTD
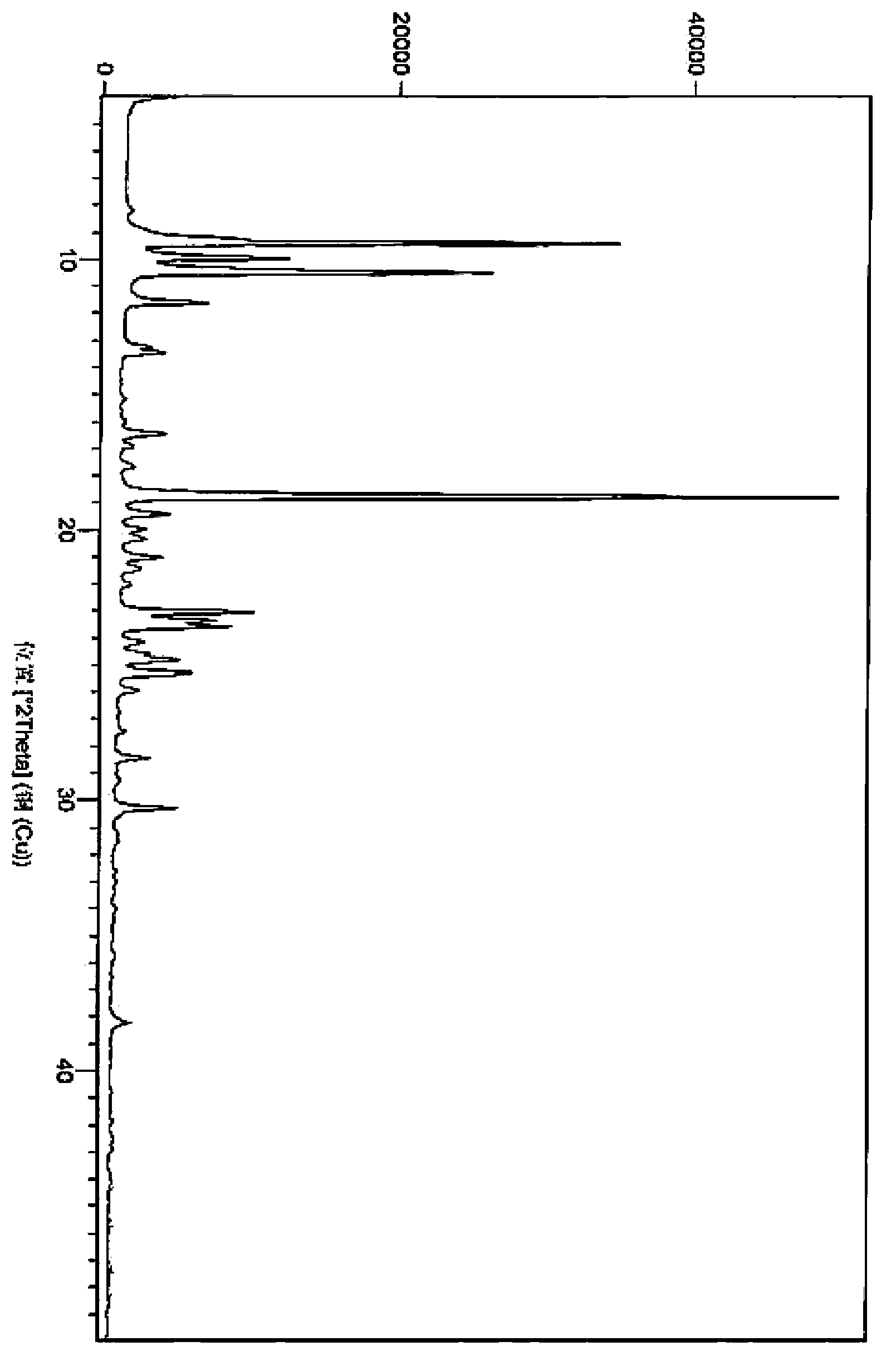
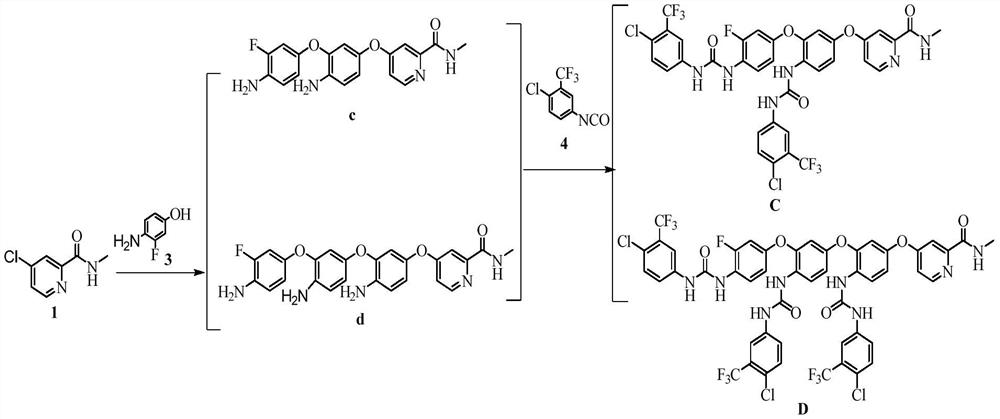
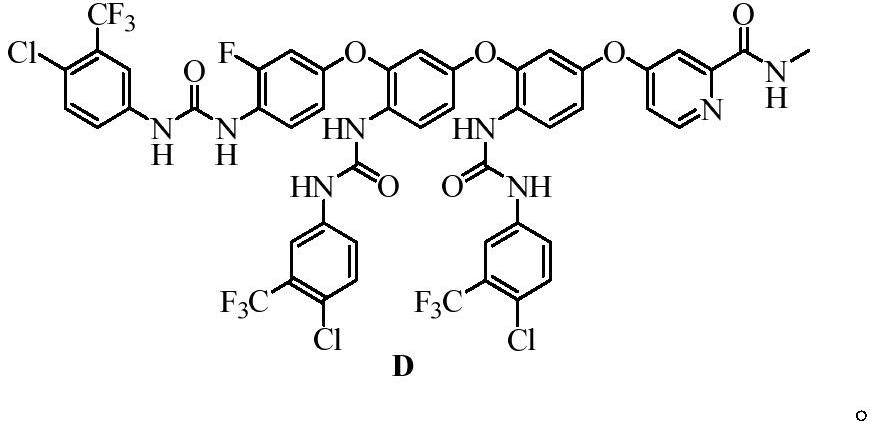
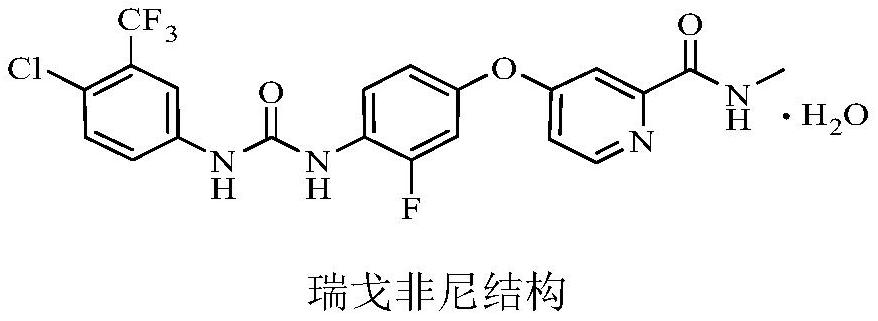
Regorafenib related substances as well as preparation method and application thereof
PendingCN111892533AShorten the timeEase of industrial productionOrganic chemistryComponent separationPotassium tert-butoxideEngineering
The invention relates to a synthesis method of regorafenib related substances B and C. According to the method, 4-chloro-N-methylpyridine-2-formamide, 4-amino-3-fluorophenol and potassium tert-butoxide are used as starting materials, and the two related substances are obtained through one synthesis route, so time and cost are saved, and industrial production is easier.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
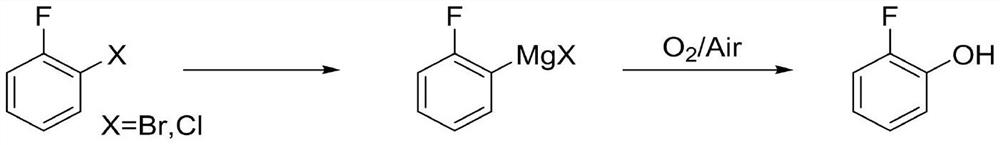
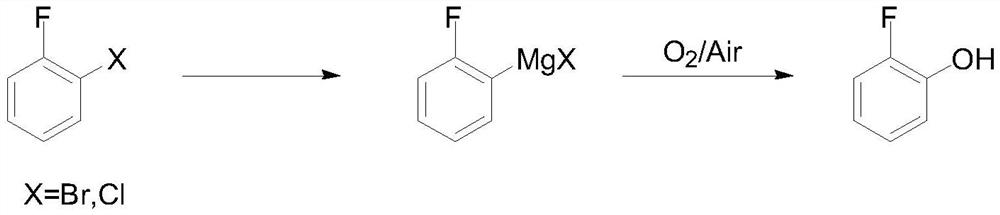
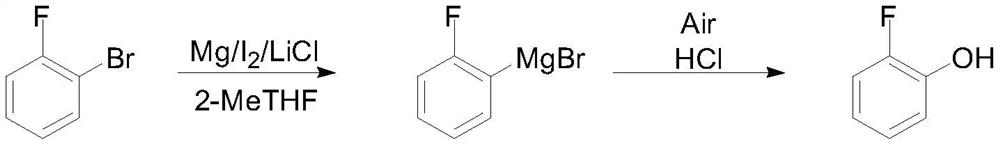
Method for preparing o-fluorophenol by one-pot method
PendingCN112645801AReduce internal eliminationReduce generationOrganic compound preparationMagnesium organic compoundsGrignard reagentOrganic synthesis
The invention discloses a method for preparing o-fluorophenol by a one-pot method, and belongs to the technical field of organic synthesis. O-bromine / chlorofluorobenzene is used as a raw material, a 2-fluorophenyl Grignard reagent is obtained through magnesium metal or Grignard reagent exchange in the presence of a stabilizer, and then oxygen or compressed air is introduced to obtain the o-fluorophenol. The method is simple and convenient in reaction operation, less in three wastes, high in yield and free of isomers, inhibits elimination of a Grignard reagent due to addition of the stabilizer, and accords with industrial amplification prospects.
Owner:甘肃瀚聚药业有限公司
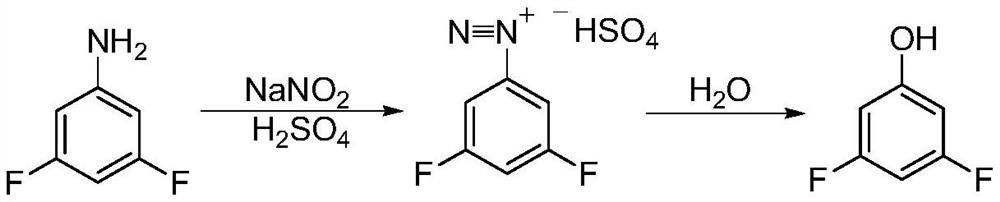
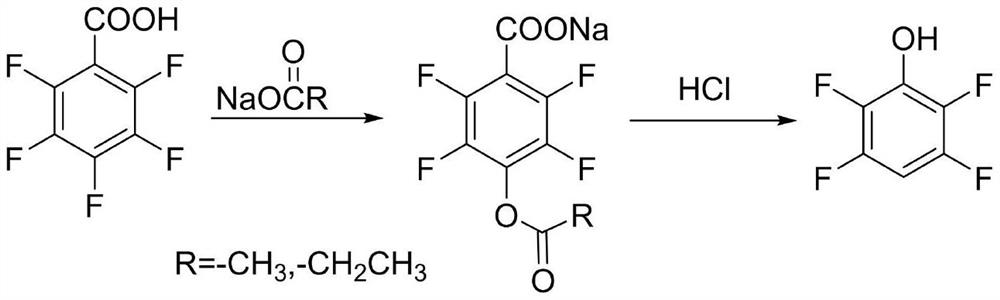
Synthesis method of 3, 5-difluorophenol
ActiveCN112608220ARaw materials are cheap and easy to getShort synthetic stepsOrganic chemistryOrganic compound preparationChemical synthesisBenzoic acid
The invention discloses a synthetic method of 3, 5-difluorophenol, and belongs to the technical field of chemical synthesis. According to the method, 3,5-difluorophenolate is obtained through a one-pot reaction of 2,4,6-trifluorobenzoic acid in a solvent under the action of alkali, 3,5-difluorophenol is obtained after acid regulation and dissociation, and the method has the advantages of cheap and easily available raw materials, short synthesis steps, simple operation, mild reaction conditions, high synthesis yield, good product quality, suitability for industrial production and the like. According to the method, cheap and easily available pentachloronitrile is taken as a raw material, 2, 4, 6-trifluoro-3, 5-dichlorobenzonitrile is obtained through a fluorination reaction, 2, 4, 6-trifluoro-3, 5-dichlorobenzoic acid is obtained through a hydrolysis reaction, and finally, the raw material 2, 4, 6-trifluoro-3, 5-dichlorobenzoic acid is synthesized through a selective dechlorination reaction, so that simple, cheap and efficient preparation of the raw material 2, 4, 6-trifluorobenzoic acid is realized, and the industrial application value of the synthesis process is improved.
Owner:ZHEJIANG ZHONGXIN FLUORIDE MATERIALS CO LTD
Improved synthesis technology of 3,4,5-trifluorophenol
ActiveCN110981699AReaction is easy to controlRealize industrial productionOrganic compound preparationGroup 3/13 element organic compoundsOrganic synthesisPhenol
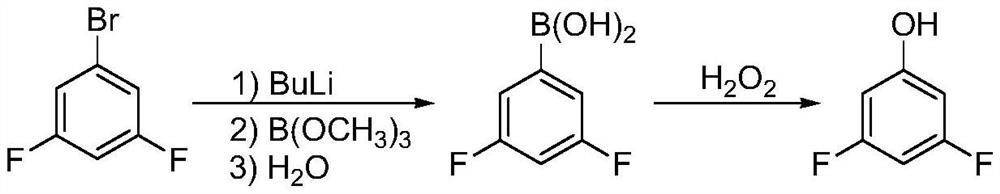
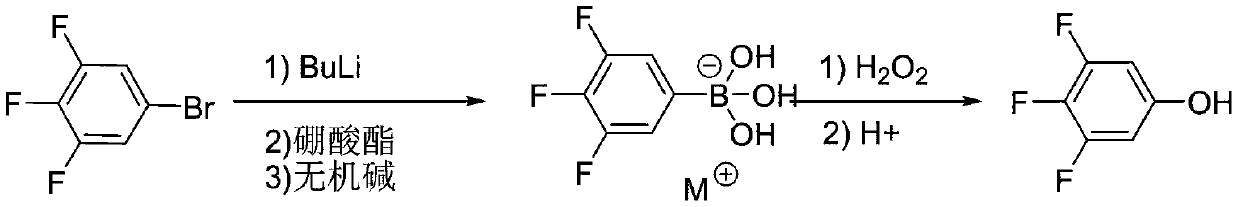
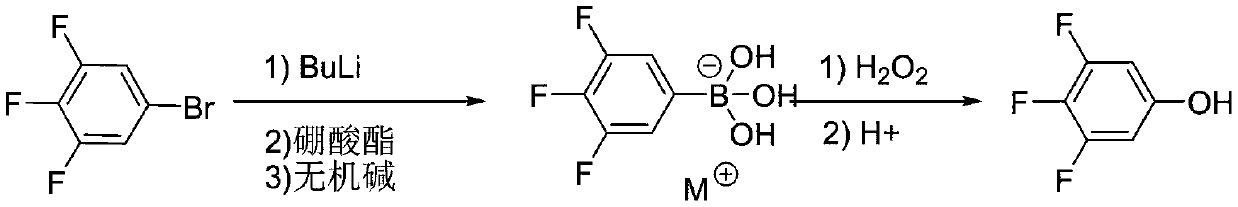
The invention discloses a synthesis technology of 3,4,5-trifluorophenol, and belongs to the technical field of organic synthesis. The method comprises the following steps: 3,4,5-trifluorobromobenzeneused as a raw material is lithiated by using n-butyllithium under an ultralow temperature condition, and then reacts with boric acid ester, an alkali is added for quenching to obtain 3,4,5-trifluorophenylborate, the 3,4,5-trifluorophenylborate is oxidized in the presence of hydrogen peroxide, and then undergoes acidolysis to obtain 3,4,5-trifluorophenol, and the obtained 3,4,5-trifluorophenol is simply distilled to obtain the product having a purity reaching 99.9% or above, having a defluorination matter content of below 50 ppm and meeting the requirements of electronic products. The technology adopting lithiation / boronation and the oxidation reaction in a water system has the advantages of mild reaction conditions, few defluorination byproducts, high product purity, and easiness in realization of industrial production.
Owner:BENGBU CHINA SYNCHEM TECH CO LTD
Method for continuously preparing pentafluorophenol by micro-reactor
ActiveCN111072455AHigh reaction yieldAvoid hydrolysisOrganic chemistryOrganic compound preparationHexafluorobenzeneMicroreactor
The invention relates to a method for continuously preparing pentafluorophenol by a microreactor, which belongs to the field of chemical production processes. The method comprises the following steps:simultaneously pumping hexafluorobenzene and an inorganic alkali aqueous solution into a micro-channel reactor by using a metering pump respectively, and mixing for a hydrolysis reaction; keeping thetemperature of the reactor at 130-170 DEG C, controlling and maintaining the pressure of a pipeline at 0.5-1.0 Mpa by an outlet quantitative pressure control valve, connecting an outlet with a heat exchanger, obtaining an aqueous solution of pentafluorophenolate from the outlet of the heat exchanger, adding hydrochloric acid steam, distilling to obtain an oil layer, and rectifying and dehydratingthe oil layer to obtain pentafluorophenol. The method is simple in process, low in cost, free of amplification effect and capable of achieving continuous production.
Owner:DALIAN QIKAI MEDICAL TECH
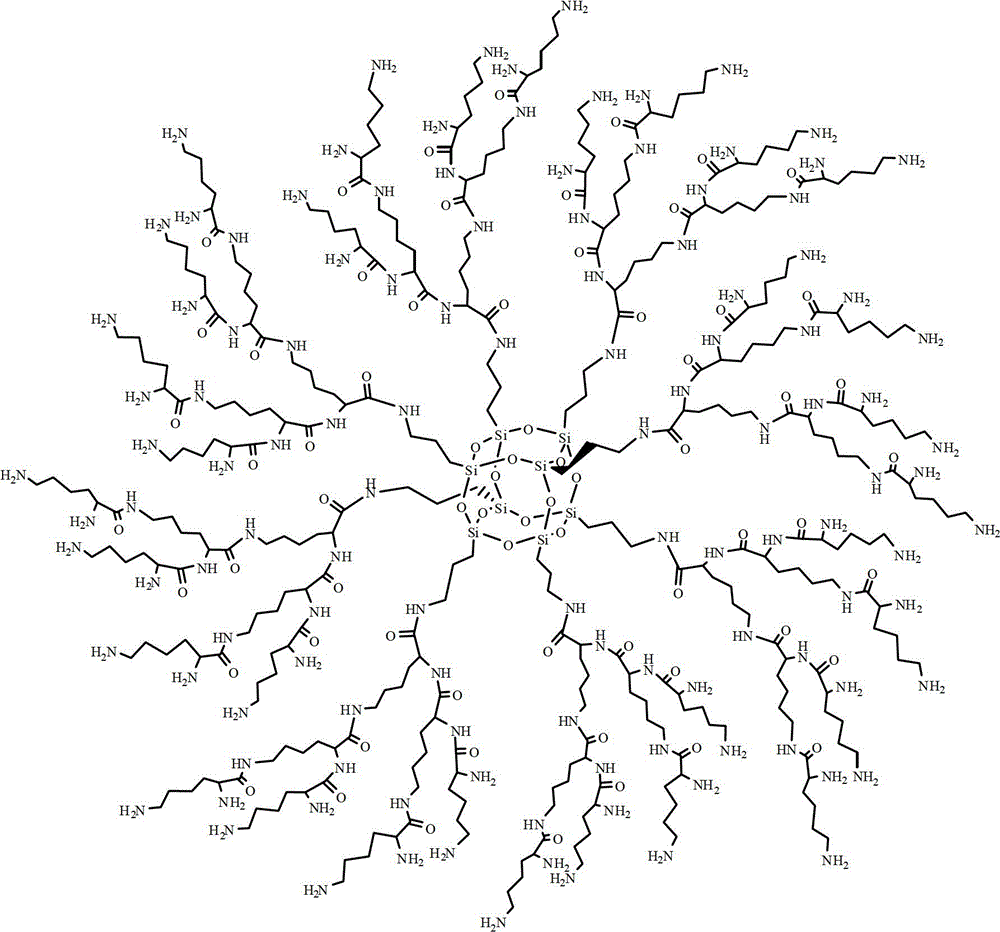
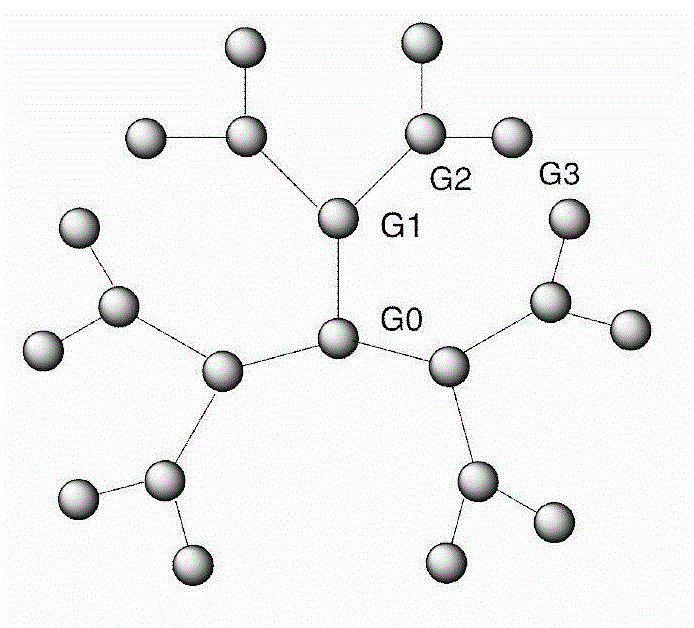
Synthesis method of dendriform compound trifluoroacetate using cage-type octamer (gamma-aminopropyl)silsesquioxane as core
InactiveCN103059317AReduce dosageReduce typesTert-Butyloxycarbonyl protecting groupPerfluoroacetic Acid
The invention discloses a method for synthesizing dendriform compound trifluoroacetate using cage-type octamer (gamma-aminopropyl)silsesquioxane as a core, which comprises the following steps: dissolving OAS hydrochloride and DIPEA (diisopropylethylamine) in DMF (N,N-dimethylformamide), dropwisely adding N,N'-di-tert-butoxycarbonyl-L-lysine N-succinimide ester or N,N'-di-tert-butoxycarbonyl-L-lysine penta-fluorophenol ester dissolved in DMF, and reacting for 10-14 hours; adding -2-2 DEG C acetonitrile, filtering, and drying for 20-24 hours to obtain tert-butoxycarbonyl-protected G1(OL); adding the G1(OL) into -2-2 DEG C trifluoroacetic acid to react for 3-5 hours, adding the reaction mixture into -2-5 DEG C aether, mixing, standing, filtering, and collecting the precipitate, thereby obtaining G1(OL) trifluoroacetate; and adjusting the proportion of the reaction materials, and repeating the rest of steps to obtain G3(OL) trifluoroacetate. The invention has the advantages of low pollution, short reaction time, simple after-treatment and high yield.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Method for synthesizing 2,3,4,5,6-pentafluorophenol
ActiveCN103787839BReduce usageLow costOrganic compound preparationEther preparation by ester reactionsPalladium on carbonPtru catalyst
Owner:SUZHOU HIGHFINE BIOTECH
A kind of method for preparing pentafluorophenol
ActiveCN107011126BHigh yieldSmooth responseOrganic chemistryOrganic compound preparationHexafluorobenzeneDodecane
The invention provides a pentafluorophenol preparation method. The pentafluorophenol preparation method is characterized by comprising the following preparation steps: adding 500-1000 parts of water, 50-100 parts of sodium hydroxide, 3-10 parts of a hydrolysis catalyst, 100 parts of hexafluorobenzene and 0.1-1 part of dodecyl secondary amine into a high pressure kettle according to parts by weight, sealing the high pressure kettle, raising the temperature to 110-150 DEG C, reacting for 3-8 hours, cooling to the room temperature, filtering, acidizing, extracting with methyl tert-butyl ether, combining an organic phase, distilling to recycle a solvent, and obtaining a crude product of 2,3,4,5,6-pentafluorophenol, adsorbing the crude product of pentafluorophenol in a crude product tank through five rectifying columns, and obtaining a purified product of pentafluorophenol.
Owner:衢州乾达科技有限公司 +1
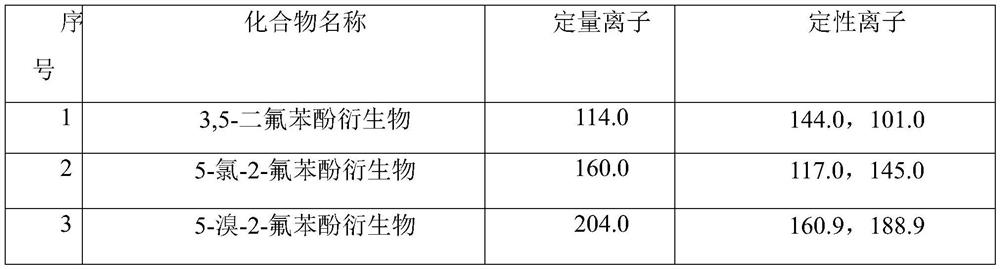
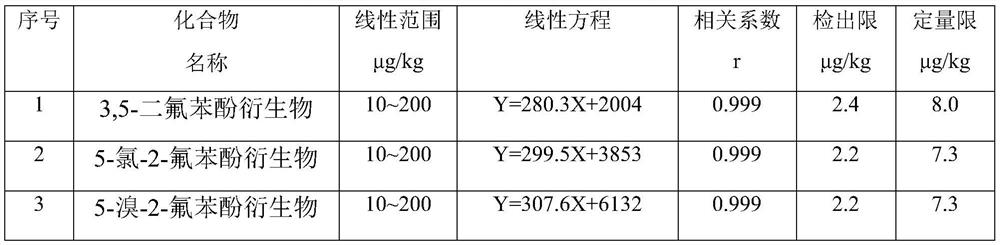
A gas chromatography-mass spectrometry method for the determination of three trace polyhalogenated phenols in textiles
The invention relates to a method for analyzing and detecting trace harmful substances, in particular to a gas chromatography-chromatographic method for determining trace amounts of 3,5-difluorophenol, 5-chloro-2-fluorophenol and 5-bromo-2-fluorophenol in textiles Mass Spectrometry. The steps of the method include enriching the target compound in the textile with a novel adsorbent p-aminobenzenesulfonate-magnesium-aluminum hydrotalcite, using an acid-dissolved adsorbent to achieve complete elution of the target compound, and efficiently extracting the compound with a small dose of organic solvent. After derivatization, it is quickly analyzed and determined by gas chromatography-mass spectrometry. The new adsorbent adopted in this method adopts the method of dispersed solid phase extraction to realize fast and efficient adsorption of the target substance, which has the advantages of fast and high efficiency; the application of acid-dissolved adsorbent can realize the complete desorption of the target substance; only a small amount of organic solvent is used as The extraction solvent of the target object has the characteristics of simple operation steps compared with the conventional phase transfer derivatization method.
Owner:丁立平 +1
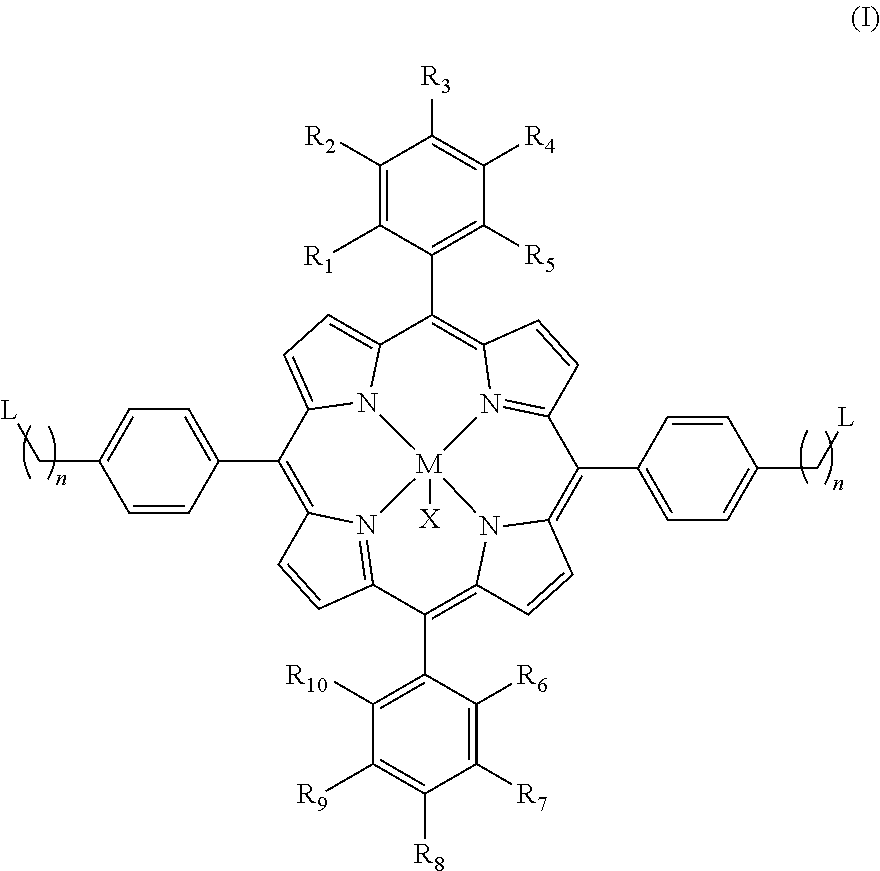
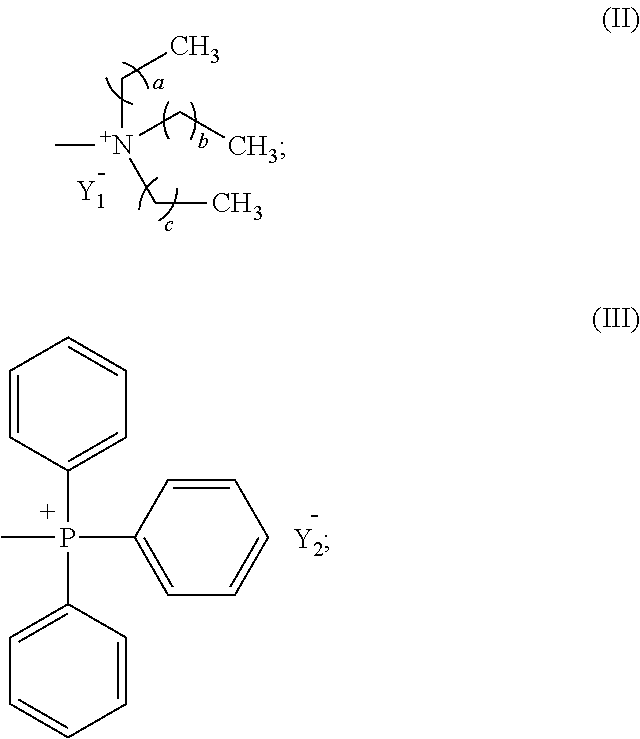
Metalporphyrin complex, preparation method therefor and method for preparing polycarbonate
ActiveUS20160194346A1High catalytic activityIron group organic compounds without C-metal linkagesGroup 3/13 element organic compoundsPhosphoniumPorphyrin
The present invention provides a metalporphyrin complex having structure represented by formula (I), wherein R1, R2, R3, R4, R5, R6, R7, R8, R9 and R10 are independently selected from one of hydrogen, halogen, aliphatic group, substituted heteroaliphatic group, aryl and substituted heteroaryl; n is 1-6; L is quaternary ammonium functional group or quaternary phosphonium functional group; M is a metal element; and X is one of halogen, —NO3, BF4—, —CN, p-methyl benzoate, o-nitrophenol oxygen anion, 2,4-dinitrophenol oxygen anion, 2,4,6-trinitrophenol oxygen anion, 3,5-dichlorophenol oxygen anion and pentafluorophenol oxygen anion. The metalporphyrin complex provided in the present invention has two quaternary ammonium functional groups or two quaternary phosphonium functional groups, and compared with the prior art, the metalporphyrin complex shows higher catalytic activity in catalyzing polymerization reaction of carbon dioxide and an epoxide.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
A kind of preparation method of pentafluorophenol
ActiveCN106966871BEasy to recycleInhibitionOrganic compound preparationGroup 3/13 element organic compoundsOrganic solventGrignard reagent
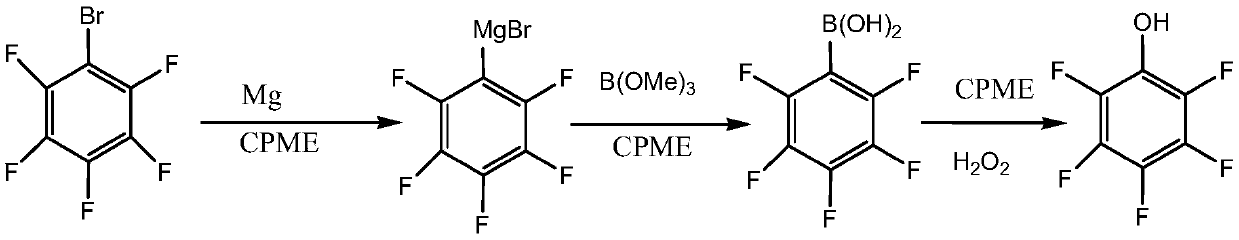
The invention relates to a preparation method for pentafluorophenol, and belongs to the technical field of the compound synthesis. The method comprises the following steps: adding Bromopentafluorobenzene to an organic solvent containing magnesium and iodine dropwise to obtain a Grignard reagent; adding the obtained Grignard reagent to the organic solvent solution of borate B(OR)3 in 20-30 DEG C dropwise, and performing the esterification reaction; enabling the obtained cyclopentyl methyl ether solution of the pentaflubenic acid to react with peroxide in 10-50 DEG C, wherein the organic solvent is the cyclopentyl methyl ether or the recycled cyclopentyl methyl ether. The preparation method is capable of using the cyclopentyl methyl ether or the recycled cyclopentyl methyl ether as the reactive solvent, and the solvent is good for the refined recycling. The provided method has the characteristics of easily obtained raw materials, stable technology, convenient operation and high product purity (greater than or equal to 99.5%).
Owner:DALIAN QIKAI MEDICAL TECH
A kind of method of synthesizing 2,3,5,6-tetrafluorophenol
ActiveCN113214050BReduce pollutionEasy to operateOrganic compound preparationCarboxylic acid esters preparationBenzoic acidPentafluorobenzoic acid
Owner:宁夏忠同生物科技有限公司
A kind of phenoxyquinoline and its synthetic method
Owner:陕西恒润化学工业有限公司
A kind of synthetic method of 3,5-difluorophenol
ActiveCN112608220BRaw materials are cheap and easy to getShort synthetic stepsOrganic chemistryOrganic compound preparationChemical synthesisBenzoic acid
The invention discloses a synthesis method of 3,5-difluorophenol, which belongs to the technical field of chemical synthesis. 2,4,6-trifluorobenzoic acid in a solvent, under the action of alkali, obtains 3,5-difluorophenolate through one-pot reaction, and obtains 3,5-difluorophenol after adjusting the acid for free. This method has raw materials It has the advantages of cheap and easy to obtain, short synthesis steps, simple operation, mild reaction conditions, high synthesis yield, good product quality, and suitable for industrial production. 2,4,6-trifluorobenzoic acid uses cheap and easy-to-obtain pentachlorobenzonitrile as a raw material, first undergoes fluorination reaction to obtain 2,4,6-trifluoro-3,5-dichlorobenzonitrile, and then undergoes hydrolysis The reaction obtains 2,4,6-trifluoro-3,5-dichlorobenzoic acid, which is finally synthesized by selective dechlorination reaction, realizing the simple, cheap and efficient preparation of raw material 2,4,6-trifluorobenzoic acid, Improve the industrial application value of the synthesis process.
Owner:ZHEJIANG ZHONGXIN FLUORIDE MATERIALS CO LTD
Compound Biotin-PEG3-SS-DBCO as well as preparation method and application thereof
ActiveCN114106012AEasy to prepareLow costOrganic chemistryBulk chemical productionCarboxyl radicalThio-
The invention belongs to the technical field of chemistry, and discloses a compound Biotin-PEG3-SS-DBCO as well as a preparation method and application thereof.The preparation method comprises the following steps: slowly adding 2, 2 '-dithio bipyridine into 2-aminoethanethiol hydrochloride to react to obtain 2-(2-pyridyl disulfide) ethylamine hydrochloride; adding thiohydracrylic acid into the methanol solution of the compound 2 to obtain 3-[(2-aminoethyl) dithio] propionic acid hydrochloride; the preparation method comprises the following steps: slowly adding pentafluorophenol into a dichloromethane solution of dibenzocyclooctyne-carboxyl (compound 4) and EDCI, and then adding a compound 3 and triethylamine to prepare dibenzocyclooctyne-disulfide bond-carboxyl (compound 5); the preparation method comprises the following steps: slowly adding pentafluorophenol into a dichloromethane solution of a compound 5 and EDCI, then adding biotin tripolyethylene glycol amino (a compound 7) and triethylamine to react, and purifying to obtain the compound Biotin-PEG3-SS-DBCO. The preparation method is simple and low in cost.
Owner:宜昌博仁凯润药业有限公司
Preparation method of BTK kinase inhibitor key intermediate
ActiveCN114751850AReduce usageReduce pollutionOrganic chemistry methodsBulk chemical productionPropanoic acidPtru catalyst
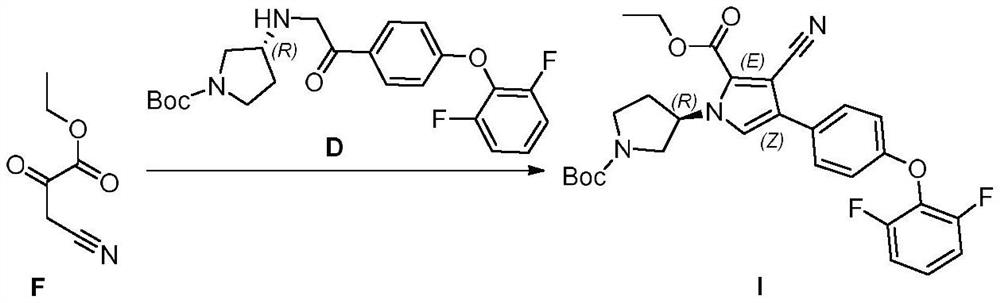
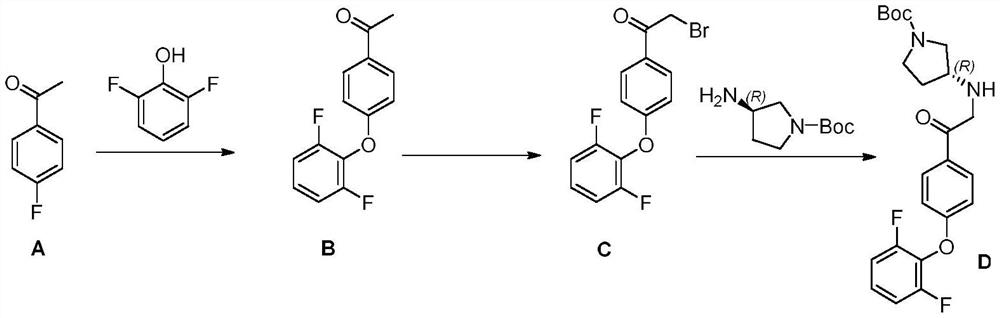
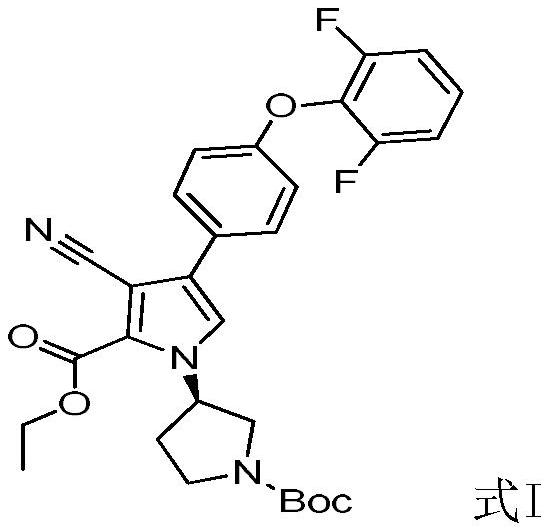
The invention relates to a preparation method of a BTK kinase inhibitor key intermediate, and belongs to the technical field of medical intermediates. The preparation method comprises the following steps: starting from p-fluoroacetophenone, carrying out substitution reaction on p-fluoroacetophenone and 2, 6-difluorophenol to obtain an intermediate B; brominating the intermediate B to obtain an intermediate C; carrying out substitution reaction on the intermediate C and (R)-1-Boc-3-aminopyrrolidine to obtain an intermediate D; the intermediate D and 3-cyano-2-oxo ethyl propionate F are subjected to a ring closing reaction, and a target compound I is obtained. Compared with the prior art, the method provided by the invention shortens the steps, improves the yield, avoids the use of a heavy metal catalyst, is beneficial to industrial expanded production, and reduces the pollution to the environment.
Owner:SHANGHAI ZAIQI BIO TECH
Screw and machining process thereof
InactiveCN112375489AImprove compactnessImprove wear resistanceCoatingsSpecial surfacesPolymer scienceFirming agent
The invention relates to the field of screws, and particularly discloses a screw and a machining process thereof. The surface of the screw is finished by a wear-resistant coating, and the wear-resistant coating comprises the following raw materials in parts by weight: 40-50 parts of polyethersulfone resin; 20-25 parts of 2,3,4,5-tetrafluorobenzamide; 15-20 parts of 4-chloro-2,6-difluorophenol; 8-10 parts of ceramic micro powder; 4-7 parts of butyronitrile; and 1-2 parts of a curing agent. The processing technology comprises the following steps: S1, preparing the wear-resistant coating; S2, spraying the wear-resistant coating; and S3, conducting gloss oil spraying. The polyether sulfone resin, 2,3,4,5-tetrafluorobenzamide and 4-chloro-2,6-difluorophenol are added, and the product obtained through the reaction of 2,3,4,5-tetrafluorobenzamide and 4-chloro-2,6-difluorophenol is coupled with the polyether sulfone resin, so that the tightness between structures is improved, and the wear resistance of the wear-resistant coating is improved; and besides, according to the machining process, gloss oil is sprayed to protect the screw, the friction coefficient of the surface of the screw is reduced, and therefore the wear-resisting effect of the surface of the screw is improved.
Owner:乐清市徐泰螺钉厂
A kind of preparation method of pentafluorophenol
ActiveCN107353181BReduce dosageLess side effectsOrganic chemistryOrganic compound preparationHexafluorobenzeneThio-
The invention provides a pentafluorophenol preparation method, which comprises: using hexafluorobenzene and potassium hydroxide as raw materials, adding an appropriate amount of tetrabutylammonium hydrogen sulfate, N-sulfopropyl-3-methylpyridine triflate, N-methyl-2-[(2-methylphenoxy)acetyl]hydrazinium methylthioamide and 2,2,6,6-tetramethylpiperidine-oxynitride to a tert-butyl alcohol aqueous solution with a mass fraction of 80-85%, heating to achieve a slight boiling state, carrying out a reflux reaction for 2-3 h, adding an appropriate amount of water, distilling to recover the tert-butyl alcohol solvent, adjusting the PH value of the remaining aqueous solution by using refined hydrochloric acid to 9-10, adsorbing with a functionalized D101 macroporous adsorption resin, acidifying with refined hydrochloric acid to achieve the PH value of 1-2, layering, distilling the upper layer water phase until no oily substance exists, collecting the distilled product, combining the collected product and the lower layer oil phase, rectifying, collecting the distillate at the temperature of 142-144 DEG C, and cooling to a room temperature to obtain the colorless and transparent crystal pentafluorophenol. According to the present invention, the preparation method has advantages of simple and reasonable process, less side reactions, high reaction yield and high product purity, and can meet the quality requirements for the preparation of the high-quality liquid crystal materials and drugs.
Owner:QUZHOU UNIV
A kind of preparation method of pentafluorophenol
ActiveCN106946659BOrganic compound preparationPreparation by rearrangement reactionsNitrosoPtru catalyst
Owner:SHANGHAI CHEMSPEC CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com