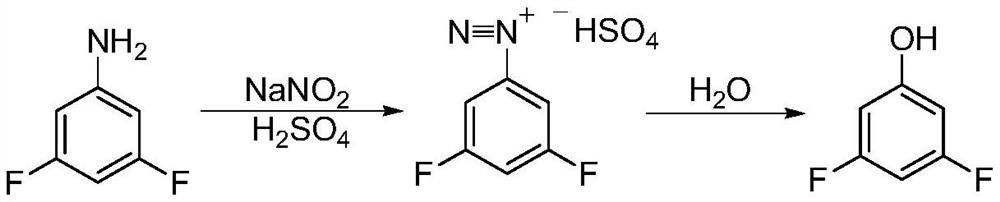
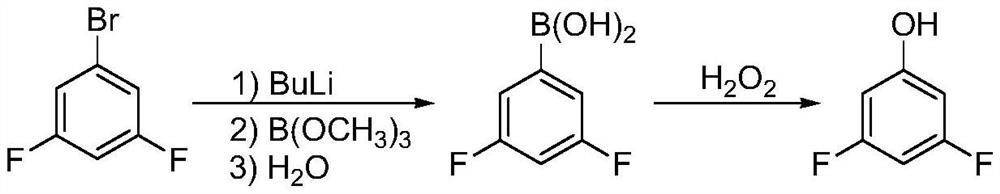
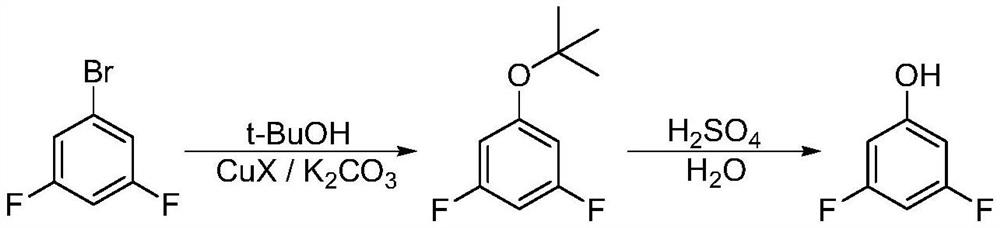
Synthesis method of 3, 5-difluorophenol
A technology of difluorophenol and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve problems such as unsatisfactory yield, high price and difficult availability, large amount of waste water, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In a 1-liter pressure-resistant reactor, add 300 grams of water, 300 grams of xylene, and 113 grams of sodium carbonate, stir at room temperature, add 75 grams of 2,4,6-trifluorobenzoic acid, seal the reactor, and heat up to 160-165°C The reaction was stirred for 10 hours, and the reaction was stopped. The reaction system was lowered to room temperature, the reaction solution was taken out, the pH was adjusted to 1-2 with concentrated hydrochloric acid, the layers were allowed to stand, the organic phase was separated, the aqueous phase was extracted with xylene, the organic phases were combined, dried, concentrated, and rectified to obtain 51.81 g of 3,5-difluorophenol, yield 93.5%, purity 99.7%.
Embodiment 2
[0045] In a 1-liter pressure-resistant reactor, add 480 grams of water and 110 grams of sodium hydroxide, stir at room temperature, add 160 grams of 2,4,6-trifluorobenzoic acid, seal the reactor, raise the temperature to 150-155°C and stir for 15 hours , stop responding. The reaction system was lowered to room temperature, the reaction solution was taken out, stirred at room temperature, adjusted to strong acidity with 10% hydrochloric acid, extracted with chloroform, combined organic phases, dried, concentrated, and rectified to obtain 3,5-difluorophenol 111.46 grams, yield 94.3%, purity 99.6%.
Embodiment 3
[0047] In a 1-liter pressure-resistant reactor, add 210 grams of water, 280 grams of toluene, and 110 grams of potassium hydroxide, stir at room temperature, add 70 grams of 2,4,6-trifluorobenzoic acid, seal the reactor, and heat up to 80-85°C Stir the reaction for 30 hours, raise the temperature to 140-145° C. and continue the reaction for 10 hours, then stop the reaction. The reaction system was lowered to room temperature, the reaction liquid was taken out, the pH was adjusted to 1-2 with 50% sulfuric acid solution, the layers were allowed to stand, the organic phase was separated, the aqueous phase was extracted with toluene, the organic phases were combined, dried, concentrated, and rectified. 49.38 g of 3,5-difluorophenol was obtained with a yield of 95.5% and a purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com