Improved synthesis technology of 3,4,5-trifluorophenol
A technology for the synthesis of trifluorophenol, which is applied in the field of improvement of the synthesis process of 3,4,5-trifluorophenol, which can solve problems such as high equipment requirements, hidden dangers, and difficult control of the reaction, and achieve the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
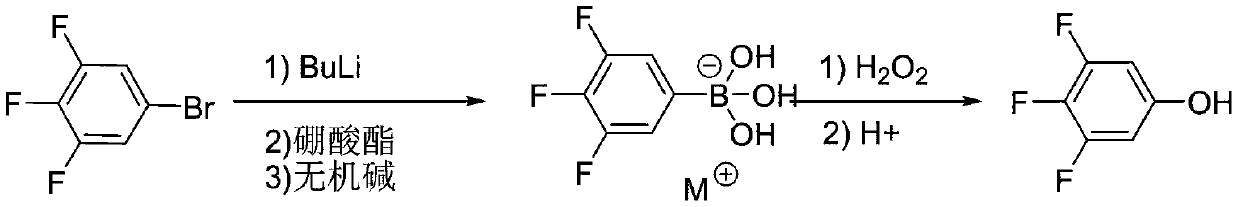
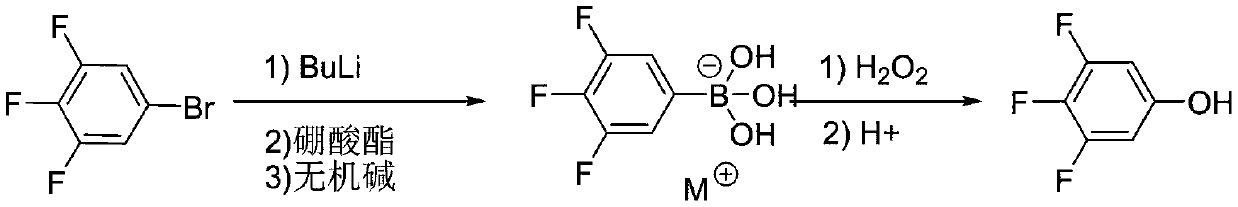
[0030] The first step: 1000mL four-neck flask, equipped with thermometer and condenser, under the protection of nitrogen, put in 3,4,5-trifluorobromobenzene 105.5g (0.5mol), tetrahydrofuran 470mL, turn on the stirring, and cool the low temperature bath to -85 ~-75°C, slowly add 220 mL (0.55 mol) of n-butyl lithium n-hexane solution (2.5 mol / L) dropwise, after the dropwise addition is completed, control the temperature and stir for 2.0 hours. At the end of heat preservation, control the temperature from -85 to -75°C to start adding 77.9 g (0.75 mol) of trimethyl borate dropwise, and keep the temperature for 2.0 hours after the dropping. Sample delivery liquid phase detection 3,4,5-trifluorobromobenzene raw material residue <0.5%. After the reaction is completed, desolvate tetrahydrofuran under negative pressure until the temperature of the reaction flask reaches 50℃ and no flow, stop desolventization, cool to 20-25℃, add 630mL pure water, control the temperature at 20-25℃, add 3...
Embodiment 2
[0033] The first step: 1000mL four-neck flask, equipped with thermometer and condenser, under the protection of nitrogen, put in 3,4,5-trifluorobromobenzene 105.5g (0.5mol), 2-methyltetrahydrofuran 490mL, start stirring, low temperature bath The temperature was lowered to -70°C to -60°C, and 220 mL (0.55 mol) of a cyclohexane solution (2.5 mol / L) of n-butyl lithium was slowly added dropwise. After the dropwise addition was completed, the temperature was controlled and stirred for 2.0 hours. At the end of the heat preservation, control the temperature from -85 to -75°C to start adding 109.5 g (0.75 mol) of triethyl borate dropwise, and keep the temperature for 2.0 hours after the dropping. Sample delivery liquid phase detection 3,4,5-trifluorobromobenzene raw material residue <0.5%. After the reaction is completed, desolve 2-methyltetrahydrofuran under negative pressure until the temperature of the reaction flask reaches 50℃ and no flow, stop desolventization, cool to 20-25℃, ad...
Embodiment 3
[0036] The first step: 1000mL four-neck flask, equipped with thermometer and condenser, under the protection of nitrogen, put in 3,4,5-trifluorobromobenzene 105.5g (0.5mol), tetrahydrofuran 470mL, turn on the stirring, and cool the low temperature bath to -70 At ~-60°C, 220 mL (0.55 mol) of a cyclohexane solution (2.5 mol / L) of n-butyl lithium was slowly added dropwise. After the addition was completed, the temperature was controlled and stirred for 2.0 hours. At the end of the heat preservation, control the temperature from -85°C to -75°C to start adding 141.0g (0.75mol) isopropyl borate dropwise, and keep the temperature for 2.0 hours after the dropping. Sample delivery liquid phase detection 3,4,5-trifluorobromobenzene raw material residue <0.5%. After the reaction is completed, desolvate tetrahydrofuran under negative pressure until the temperature of the reaction flask reaches 50℃ and no flow, stop desolventization, cool to 20-25℃, add 630mL pure water, control the tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com