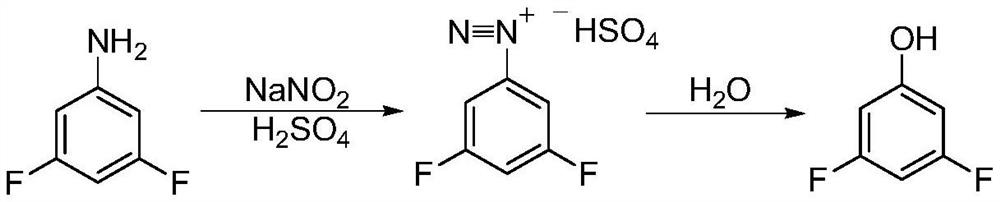
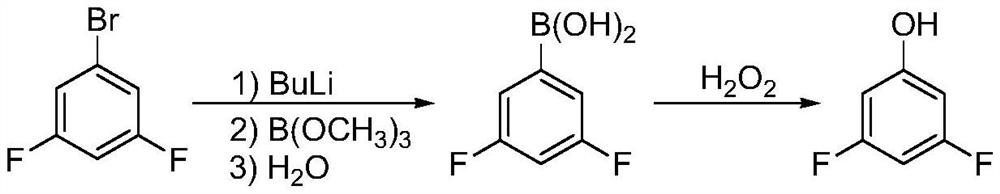
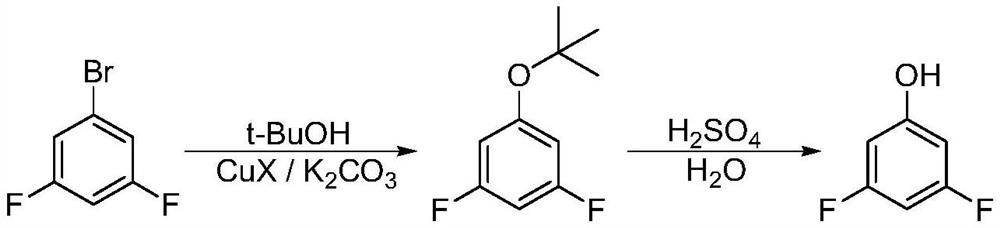
A kind of synthetic method of 3,5-difluorophenol
A technology for difluorophenol and a synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of expensive and difficult to obtain raw materials, high price and difficult to obtain, and unsatisfactory yield, and achieves improvement The effect of industrial application value, efficient preparation and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In a 1-liter pressure-resistant reaction kettle, add 300 grams of water, 300 grams of xylene, and 113 grams of sodium carbonate, stir at room temperature, add 75 grams of 2,4,6-trifluorobenzoic acid, seal the reaction kettle, and heat up to 160-165°C The reaction was stirred for 10 hours, and the reaction was stopped. The reaction system was cooled to room temperature, the reaction solution was taken out, the pH was adjusted to 1-2 with concentrated hydrochloric acid, left to stand for layers, the organic phase was separated, the aqueous phase was extracted with xylene, the organic phases were combined, dried, concentrated and rectified to obtain 51.81 g of 3,5-difluorophenol, yield 93.5%, purity 99.7%.
Embodiment 2
[0045] In a 1-liter pressure-resistant reaction kettle, add 480 grams of water and 110 grams of sodium hydroxide, stir at room temperature, add 160 grams of 2,4,6-trifluorobenzoic acid, seal the reaction kettle, and heat up to 150-155 °C and stir for 15 hours , stop the reaction. The reaction system was cooled to room temperature, the reaction solution was taken out, stirred at room temperature, adjusted to pH strongly acidic with 10% hydrochloric acid, extracted with chloroform, combined with organic phases, dried, concentrated and rectified to obtain 3,5-difluorophenol 111.46 g, yield 94.3%, purity 99.6%.
Embodiment 3
[0047] In a 1-liter pressure-resistant reaction kettle, add 210 grams of water, 280 grams of toluene, and 110 grams of potassium hydroxide, stir at room temperature, add 70 grams of 2,4,6-trifluorobenzoic acid, seal the reaction kettle, and heat up to 80-85°C The reaction was stirred for 30 hours, the temperature was raised to 140-145° C., the reaction was continued for 10 hours, and the reaction was stopped. The reaction system was lowered to room temperature, the reaction solution was taken out, adjusted to pH 1-2 with 50% sulfuric acid solution, left to stand for layers, the organic phase was separated, the aqueous phase was extracted with toluene, the organic phases were combined, dried, concentrated and rectified, 49.38 g of 3,5-difluorophenol were obtained with a yield of 95.5% and a purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com