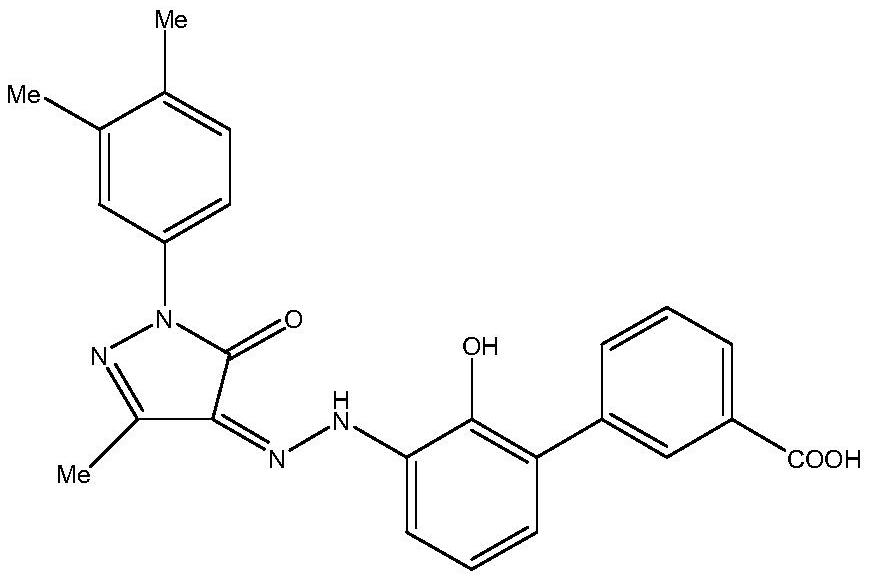
Method for synthesizing Eltrombopag using microchannel reactor
A microchannel reactor and reaction technology, applied in chemical instruments and methods, chemical/physical/physical chemical reactors, organic chemistry, etc., can solve problems such as easy decomposition, reduce side reactions, increase overall yield and Product quality, effect of inhibiting decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
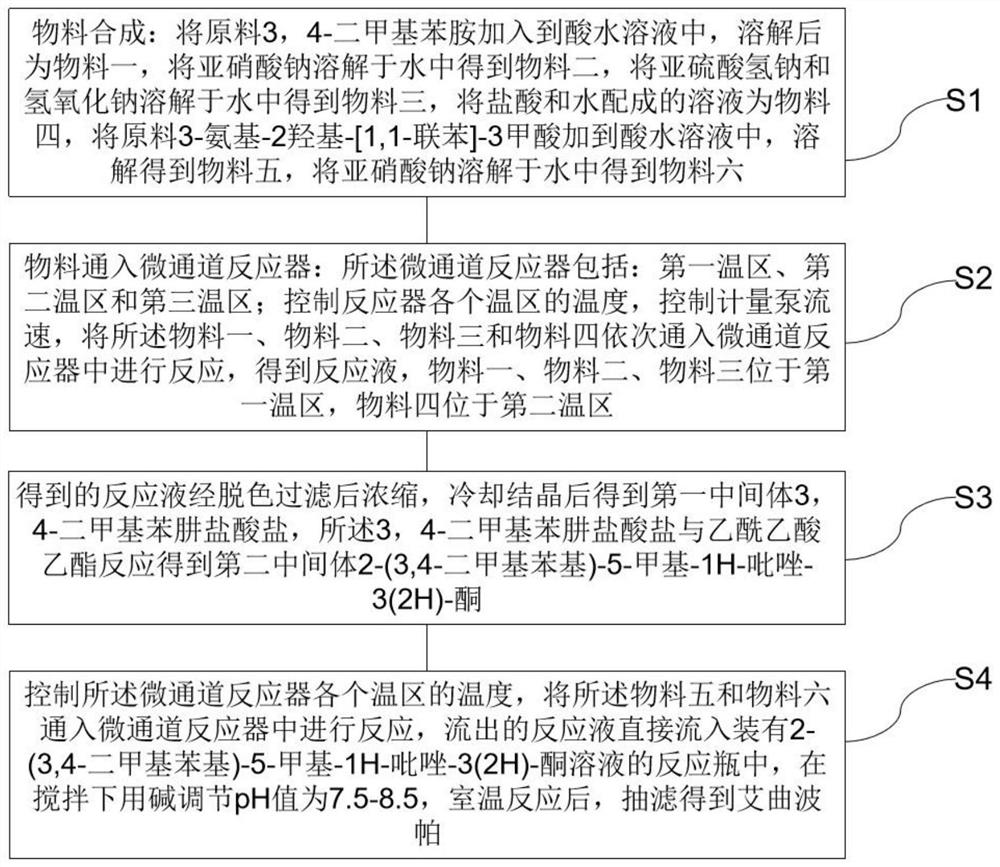
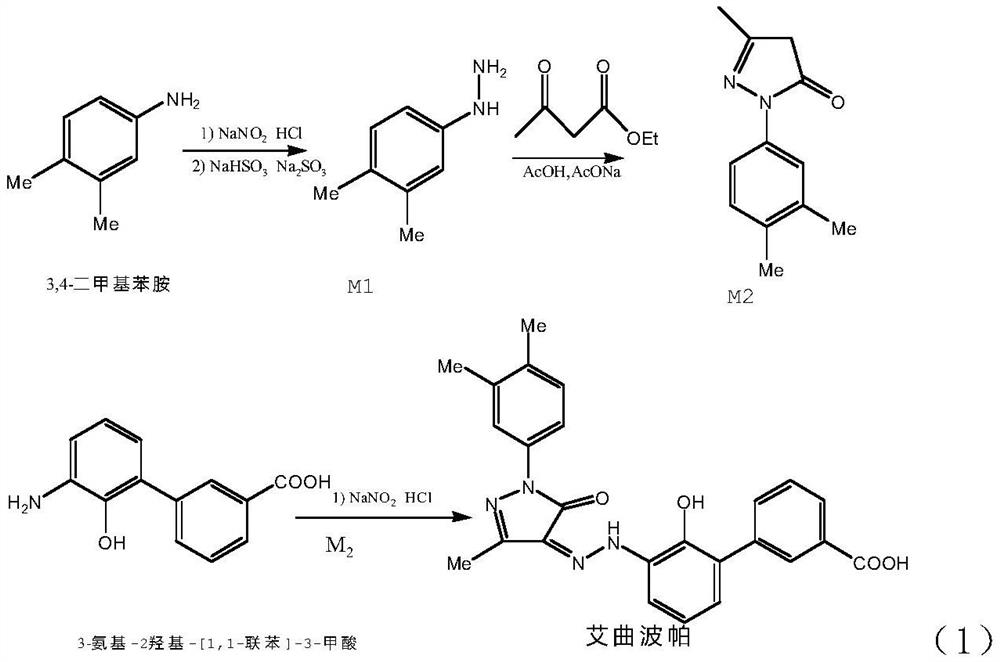
[0053] ①Put 36.7g of 3,4-dimethylaniline into 90g of concentrated hydrochloric acid and 220g of water, stir and dissolve to obtain material 1.
[0054] ②Put 21.9g of sodium nitrite into 178g of water, stir and dissolve to obtain material 2.
[0055] ③Put 38g of sodium bisulfite and 13g of sodium hydroxide into 200g of water, stir and dissolve to obtain material three.
[0056] ④ Add 100 g of concentrated hydrochloric acid into 50 g of water and stir to obtain material 4.
[0057] ⑤ Control the temperature of the first temperature zone (the first, second, third, fourth and fifth reaction modules) to be 5°C, and the temperature of the second temperature zone (the sixth, seventh, eighth and ninth reaction modules) The temperature is 85°C, and the temperature in the third temperature zone (the tenth reaction module) is 25°C.
[0058] ⑥ The material flow rate of 20g / min is injected into the first reaction module by the pump, and the material two flow rate of 7.9g / min is injected ...
Embodiment 2
[0061] This embodiment and embodiment 1 are different from the diazonium reaction temperature in the first temperature zone described in step ⑤ of this embodiment is 10 ℃, and the 3,4-dimethylphenylhydrazine hydrochloride obtained is 46.2g, and the yield is 88.3%, HPLC purity 99.3%.
Embodiment 3
[0063] This embodiment is different from Example 1 in that the diazonium reaction temperature in the first temperature zone described in step ⑤ of this embodiment is 20 ° C, and 43.7 g of 3,4-dimethylphenylhydrazine hydrochloride is obtained, and the yield is 83.6%, HPLC purity 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com