Method for synthesizing sex pheromone of hyphantria cunea
A technology of a white moth and a synthesis method, which is applied in the field of synthesis of American moth sex pheromone, can solve the problems of side reactions, difficult to control sulfonic acid esterification reaction, inability to obtain a sulfonic acid esterified product, and the like, and achieves enantiomers. The effect of high selectivity and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
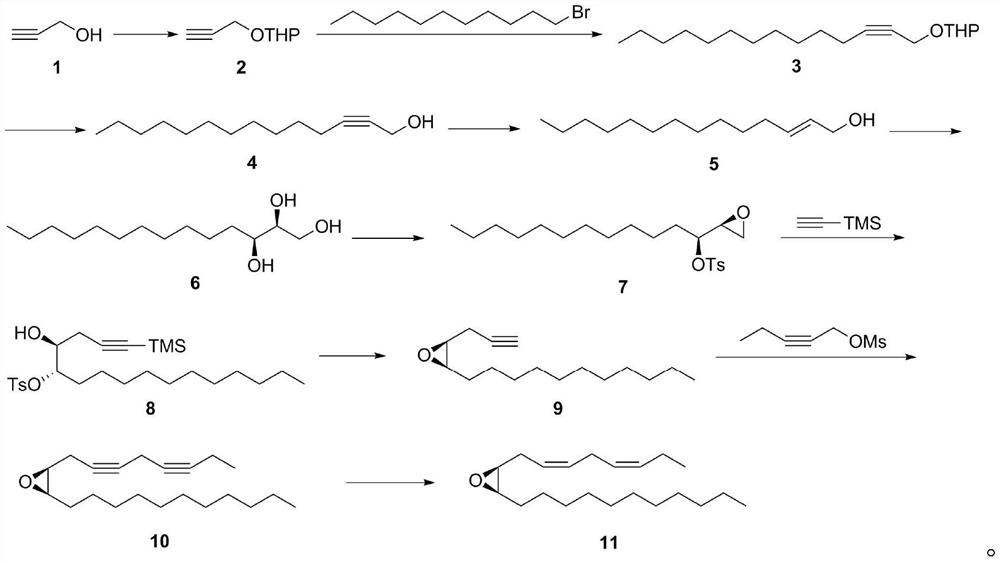
[0035] Add 0.3568mol of propynyl alcohol, 150mL of dichloromethane, and 0.03568mol of p-toluenesulfonamide into a 250mL single-necked bottle, cool in an ice bath, and slowly drop in 42.02g of dihydropyran. After completion, the reaction was quenched by adding saturated aqueous sodium bicarbonate solution, and the organic phase was extracted with dichloromethane, washed with water, washed with brine, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain compound 2 with a yield of 90%.
Embodiment 2
[0037] The PTSA in Example 1 was replaced by pyridinium p-toluenesulfonate, and other conditions remained unchanged, and the yield of compound 2 was 89%.
[0038] Step (2), the synthesis of compound 3:
Embodiment 3
[0040] Under the protection of argon, add 0.19173mol compound 2 and 250mL dry tetrahydrofuran into a 1000mL three-neck flask, cool to -78°C, add 71mL n-BuLi solution with a concentration of 2.7mol / L dropwise, and stir for half an hour after the dropwise addition , Add 0.1743mol 1-bromoundecane and 40g 1,3-dimethylpropylene urea dropwise in sequence at -78°C. After the dropwise addition is completed, slowly rise to room temperature and stir for 24 hours. Gas chromatography monitors until the end of the reaction, and the reaction is complete Finally, the reaction was quenched by adding saturated ammonium chloride aqueous solution, and the organic phase was extracted with ethyl acetate, washed with water, washed with brine, dried over anhydrous sodium sulfate, and purified by column chromatography to obtain compound 3 with a yield of 80%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com