Serum-free culture medium for in-vitro culture and amplification of bone marrow mesenchymal stem cells
A technology of serum-free medium and bone marrow mesenchyme, which is applied in the field of cell biology, can solve the problems of residual animal serum, failure to achieve serum-free culture, and inability to promote bone marrow mesenchymal stem cell adhesion, etc., to achieve no environmental pollution, Maintain biological effects and multi-lineage differentiation potential, maintain the effect of multi-lineage differentiation potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
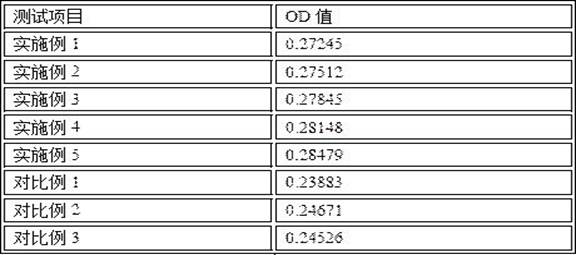
Embodiment 1
[0022] A serum-free medium for culturing and expanding bone marrow mesenchymal stem cells in vitro, characterized in that it is an aqueous solution, and its components and contents are as follows: basal medium 20g / L, growth factor 5ng / mL, Kombucha fermentation broth 8mg / mL, serum protein 3mg / mL, selenomethionine 1ng / mL, sodium ferulate 0.1mg / mL, metformin 3ng / mL, 3'-deoxynicotinamide adenine dinucleotide 5ng / mL , L-glutamine 150mg / L, multivitamin 30μM, insulin 12mU / L.
[0023] The multivitamins are formed by mixing vitamin A, vitamin B2, vitamin C, vitamin D and vitamin E in a mass ratio of 1:1:0.8:2:1.
[0024] The basal medium is DMEM-F12; the DMEM-F12 culture solution is a mixture of F12 culture solution and DMEM culture solution at a volume ratio of 1:2.
[0025] The growth factors are transforming growth factor β1 (TGF-β1), heparin-binding epidermal growth factor (HB-β), insulin growth factor-1 (IGF-1), basic fibroblast growth factor (bFGF), epidermal growth factor The ...
Embodiment 2
[0029] A serum-free medium for culturing and expanding bone marrow mesenchymal stem cells in vitro, characterized in that it is an aqueous solution, and its components and contents are as follows: basal medium 25g / L, growth factor 8ng / mL, Kombucha fermentation broth 12mg / mL, serum protein 8mg / mL, selenomethionine 3ng / mL, sodium ferulate 0.2mg / mL, metformin 4ng / mL, 3'-deoxynicotinamide adenine dinucleotide 7ng / mL , L-glutamine 250mg / L, multivitamin 50μM, insulin 17mU / L.
[0030] The multivitamins are formed by mixing vitamin A, vitamin B2, vitamin C, vitamin D and vitamin E in a mass ratio of 1.3:1:0.9:2:1.3.
[0031] The basal medium is DMEM-F12; the DMEM-F12 culture solution is a mixture of F12 culture solution and DMEM culture solution at a volume ratio of 1:2.5.
[0032] The growth factors are transforming growth factor β1 (TGF-β1), heparin-binding epidermal growth factor (HB-β), insulin growth factor-1 (IGF-1), basic fibroblast growth factor (bFGF), epidermal growth factor ...
Embodiment 3
[0036] A serum-free medium for culturing and expanding bone marrow mesenchymal stem cells in vitro, characterized in that it is an aqueous solution, and its components and contents are as follows: basal medium 30g / L, growth factor 10ng / mL, Kombucha fermentation broth 16mg / mL, serum protein 13mg / mL, selenomethionine 6ng / mL, sodium ferulate 0.25mg / mL, metformin 6ng / mL, 3'-deoxynicotinamide adenine dinucleotide 8ng / mL , L-glutamine 350mg / L, multivitamin 70μM, insulin 19mU / L.
[0037] The multivitamins are formed by mixing vitamin A, vitamin B2, vitamin C, vitamin D and vitamin E in a mass ratio of 1.5:1:1:2:1.5.
[0038] The basal medium is DMEM-F12; the DMEM-F12 culture solution is a mixture of F12 culture solution and DMEM culture solution at a volume ratio of 1:3.
[0039] The growth factors are transforming growth factor β1 (TGF-β1), heparin-binding epidermal growth factor (HB-β), insulin growth factor-1 (IGF-1), basic fibroblast growth factor (bFGF), epidermal growth factor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com