Primers and kit for detecting mycoplasma pneumoniae
A technology of mycoplasma pneumoniae and kits, which is applied in the determination/testing of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., which can solve the problems of shortened reaction time, achieve shortened detection time, high sensitivity, and improve detection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Design and screening of fluorescent ring-mediated isothermal amplification primer set for detection of Mycoplasma pneumoniae in the present invention
[0042] The primers in the present invention are designed and prepared according to the following method: using the Mycoplasma pneumoniae SDC1 gene sequence (GenBank accession No. M35024) as a template to design outer primers (F3 and B3), inner primers (FIP and BIP), and two accelerated amplification The loop primers for the amplification reaction were artificially synthesized.
[0043] The optimization and screening process of the primer set in the present invention:
[0044]In order to develop amplification primers suitable for the fluorescent LAMP system that meet clinical needs, dozens of primers with different sequences were designed for different amplification fragments, and four primers F3, B3, FIP and BIP of these primary screening primers were first synthesized. Then use standard strain DNA for amplifi...
Embodiment 2
[0073] Primer set sensitivity and specificity experiment in embodiment 2 of the present invention
[0074] After the screening in Example 1 above, the set of primers MP2 was initially selected for subsequent verification experiments.
[0075] One. Sensitivity detection of primer sets in the present invention:
[0076] 1. Detection method: extract the genomic DNA of MP standard strain FH strain (ATCC15531), and perform 10-fold equivalent dilution with TE buffer, as a template in the fluorescent LAMP reaction system. Each reaction system contains 0.6, 6, 60, 600, 6000, 60000 copies of DNA template of MP respectively. The experiment was repeated three times.
[0077] 2. Sensitivity test results of the primer set in the present invention:
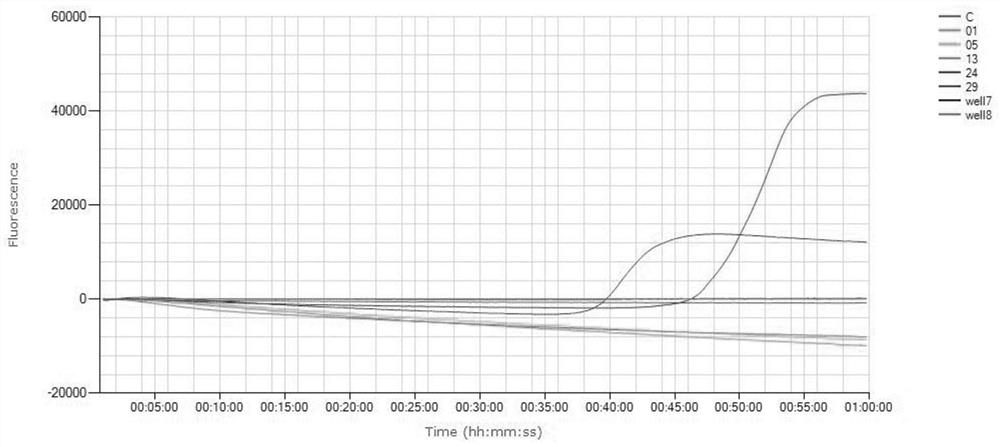
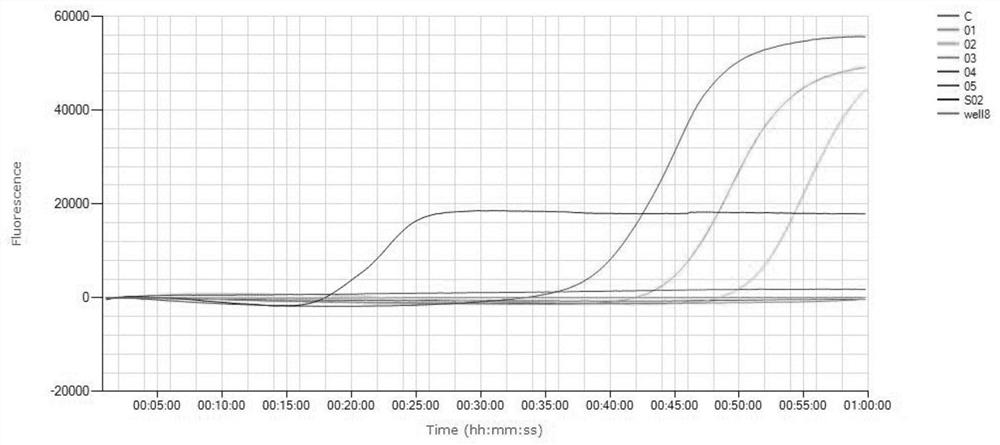
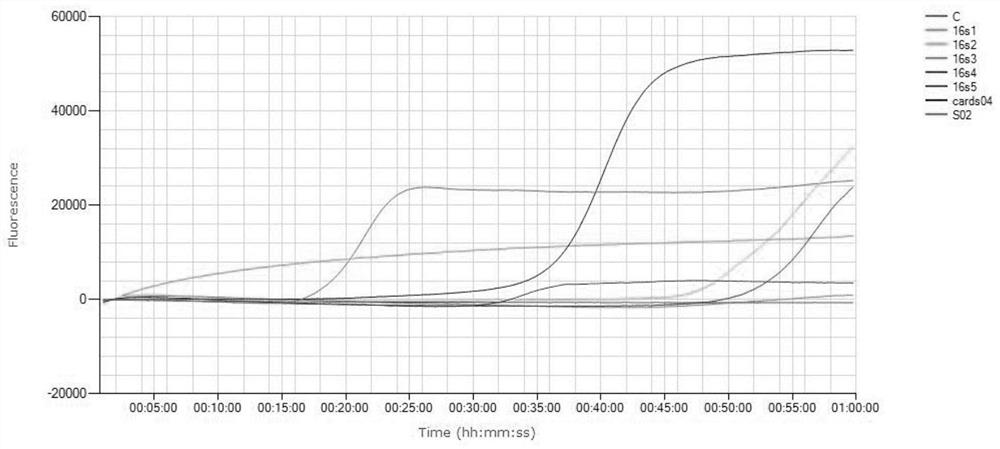
[0078] The DNA extracted and purified from the MP standard strain FH culture solution was diluted 10 times and used in the fluorescent LAMP reaction system, so that each reaction system contained 0.6, 6, 60, 600, 6000, 60000 copies respectiv...
Embodiment 3
[0083] Application of the primer set in the embodiment 3 of the present invention in the detection of Mycoplasma pneumoniae
[0084] Utilize the kit in the present invention to carry out detection verification to 247 clinical specimens.
[0085] 1. Source of samples: 247 outpatient and inpatient pediatric patients in a tertiary hospital were selected. Pharyngeal swab specimens were collected from the patients. The swabs were placed in 2ml MP transfer medium and frozen at -20°C for later use.
[0086] 2. Inclusion criteria: Fever (body temperature ≥ 38°C), cough or sore throat and other symptoms of respiratory tract infection, the course of the disease is within 7 days, mainly community-acquired pneumonia or mycoplasma pneumonia.
[0087] 3. Exclusion criteria: patients with pulmonary tuberculosis and lung tumors, non-infectious pulmonary interstitial pneumonia, pulmonary edema, atelectasis, pulmonary embolism, pulmonary eosinophilic infiltration, and immunocompromised patients...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com