Application of erythritol in preparation of medicine for treating non-alcoholic fatty liver
An erythritol, non-alcoholic technology, applied in the field of application of erythritol in the preparation of drugs for the treatment of non-alcoholic fatty liver, can solve the problems of no research and reports on the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
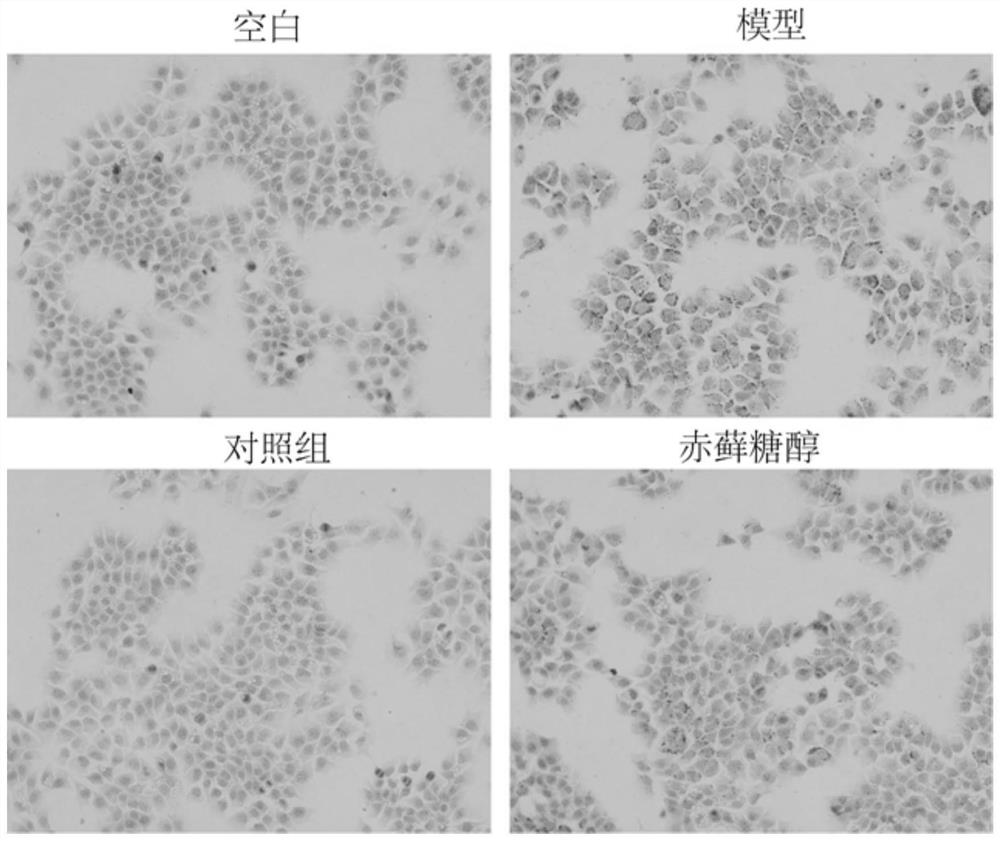
[0032] Example 1 AML12 Cell Oil Red O Staining Experiment
[0033] experimental method:
[0034] (1) AML12 cells were cultured and seeded in six-well plates. The formula of cell culture medium is F12 medium with 4.5g / L glucose, 10% fetal calf serum, 1% penicillin and streptomycin, 1% ITS, 40ng / ml dexamethasone (Sigma, D4902-100mg). Cells were cultured in 5% CO 2 and in a cell culture incubator at 37°C for 12 hours.
[0035] (2) Remove the original culture medium and replace it with F12 with only 4.5g / L glucose as the culture medium to culture the cells, and starve the AML12 cells for 3 hours.
[0036] (3) AML12 cells were co-incubated with 250 mg / ml erythritol and 1000 μM free fatty acid (oleic acid:palmitic acid=2:1) for 24 hours; among them, they were divided into blank group (F12 medium), model group (1000 μM free fatty acid ), control group (250mg / ml erythritol), experimental group (250mg / ml erythritol+1000μM free fatty acid).
[0037] (4) The cell culture medium wa...
experiment example 2W
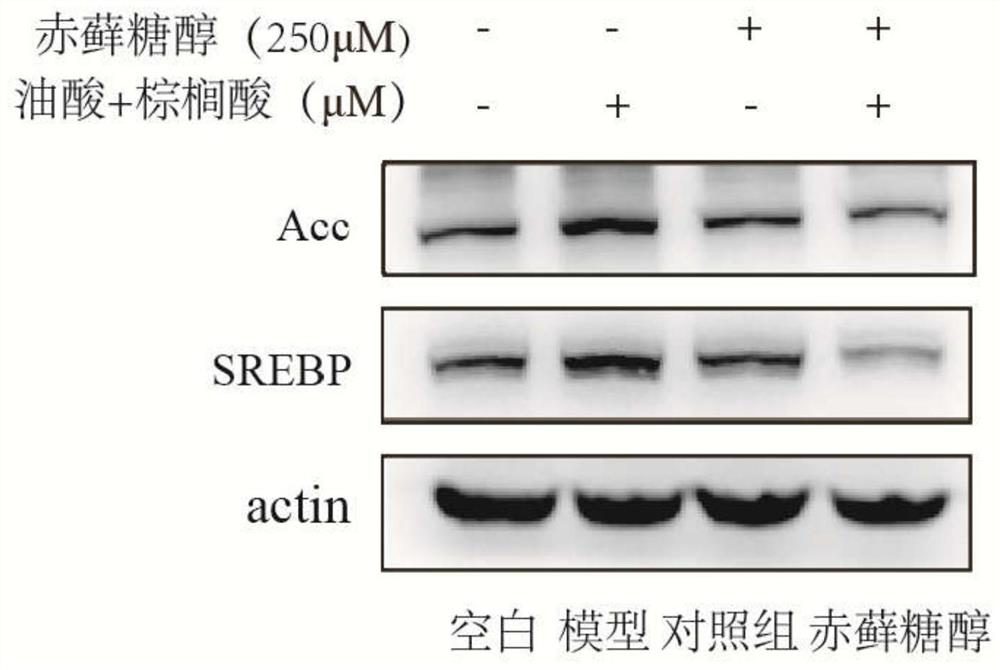
[0044] Experimental example 2Western blot detection of lipid regulatory protein expression in HEPG2 cells
[0045] experimental method
[0046] (1) Culture HEPG2 cells and inoculate them in a six-well plate with a density of 1×10 per well 6 cells. The formula of the cell culture medium is DMEM medium with 4.5g / L glucose, 10% fetal bovine serum and 1% penicillin and streptomycin. Cells were cultured in 5% CO 2 and in a cell culture incubator at 37°C for 12 hours.
[0047] (2) Remove the original culture medium and replace it with DMEM with only 4.5g / L glucose as the culture medium to culture the cells, and starve the HEPG2 cells for 3 hours.
[0048] (3) Co-incubate HEPG2 cells with 250 mg / ml erythritol and 1000 μM free fatty acid (oleic acid:palmitic acid=2:1) for 24 hours; wherein, they are divided into blank group (DMEM medium), model group (1000 μM free fatty acid ), control group (250mg / ml erythritol), experimental group (250mg / ml erythritol+1000μM free fatty acid)....
Embodiment 3
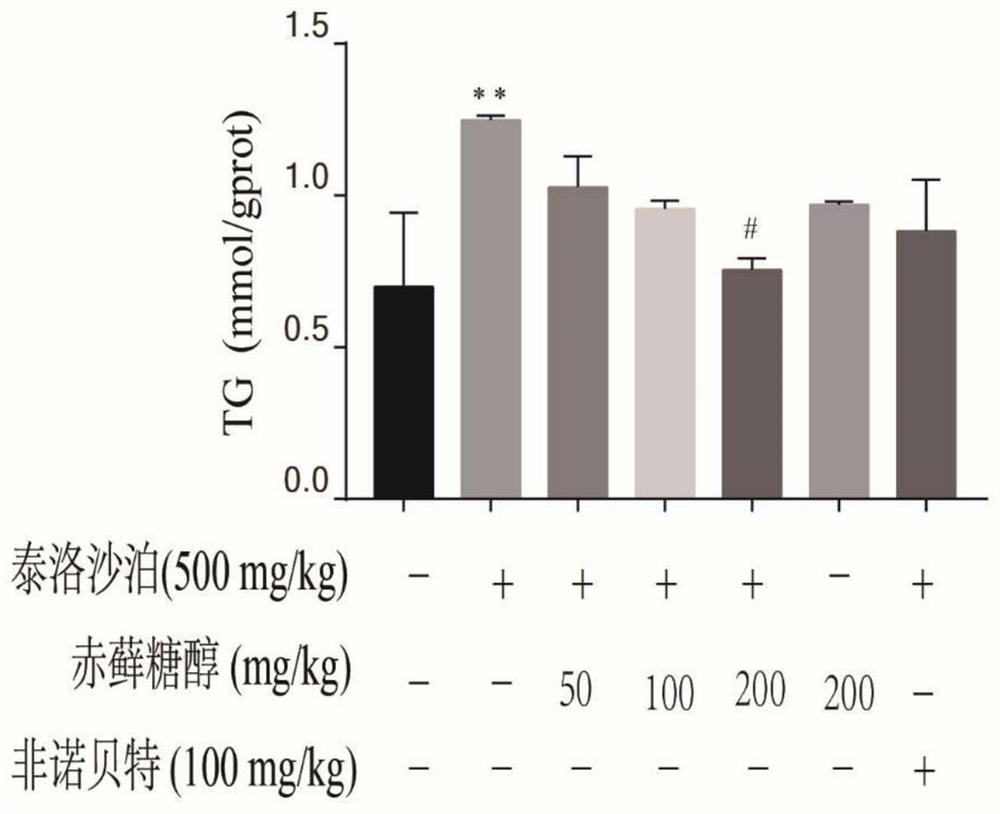
[0058] Determination of TG in the mouse liver of embodiment 3
[0059] experimental method:
[0060] (1) Raise the mice in an SPF environment with a temperature of 24±1° C. and a relative humidity of 40%-80%.
[0061] (2) Before the experiment, the mice were separated into cages and fasted for 12 hours, and then injected intraperitoneally with 50 mg / kg, 100 mg / kg, and 200 mg / kg of erythritol for 1 hour and then injected with 500 mg / kg of tyloxapol Mice were injected intraperitoneally for 12 hours. The mice were randomly divided into seven groups: blank group (normal saline), model group (500mg / kg tyloxapol), control group (200mg / kg erythritol), drug low dose group (50mg / kg erythritol sugar alcohol+500mg / kg tyloxapol), drug middle dose group (100mg / kg erythritol+500mg / kg tyloxapol), drug high dose group (200mg / kg erythritol+500mg / kg Tyloxapol), drug positive control group (100mg / kg fenofibrate+500mg / kg Tyloxapol).
[0062] (3) The mouse liver was completely removed and cut ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com