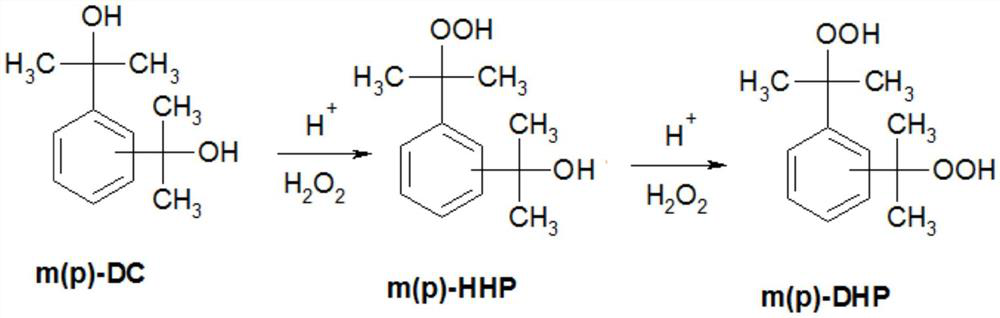
Method for preparing m-diisopropylbenzene hydrogen peroxide and p-diisopropylbenzene hydrogen peroxide
A kind of technology of m-dicumyl dihydroperoxide and dicumyl dihydroperoxide, which is applied in the field of preparing m-dicumyl dihydroperoxide and p-dicumyl dihydroperoxide, It can solve the problems of difficult separation of p-dicumyl dihydroperoxide, low yield of reaction products, equipment corrosion, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0033] According to a preferred embodiment of the present invention, the method further includes cooling the reaction mixture before solid-liquid separation, preferably, cooling the reaction mixture to 0-20°C before solid-liquid separation; more preferably , cooling the reaction mixture to 5-10° C. before solid-liquid separation.
[0034] According to the present invention, the method of solid-liquid separation is not particularly limited, and it may be a method of solid-liquid separation commonly used in the art, such as suction filtration or centrifugation.
[0035] According to the present invention, in order to improve the purity and the yield of the obtained p-dicumyl dihydroperoxide, the method may also include washing the solid phase containing p-dicumene dihydroperoxide to obtain Para-Dicumyl Dihydroperoxide Solid.
[0036] According to the present invention, the method of washing the solid phase containing p-dicumene dihydroperoxide includes washing the solid phase c...
Embodiment 1
[0044] 1) 100 milliliters of toluene, 10 grams of raw materials containing 98% by weight of m-DC and p-DC (the weight ratio of the two is 2.5:1), 17.5 grams of 50% by weight hydrogen peroxide and 0.25 gram of sulfuric acid (concentration is 98% by weight %) were mixed, started to stir, and reacted at 50°C under the condition of 0.02MPa. During the reaction, the water generated by the reaction was removed in time, and the evaporated toluene was returned to the reaction system for reuse, and the reaction was carried out for 3 hours. Gas chromatography Measure the content of m-HHP in the reaction solution to be 0.09% by weight, stop stirring, cool the reaction solution to 7°C for suction filtration, wash the obtained filter cake with a small amount of 7°C toluene and distilled water, dry the filter cake after washing, and obtain p -DHP product with a yield of 96% and a purity of 99.0%.
[0045] 2) The obtained filtrate removes the water phase to obtain an oil phase, and the oil p...
Embodiment 2
[0047] 1) 150 milliliters of toluene, 10 grams of raw materials containing 98% by weight of m-DC and p-DC (the weight ratio of the two is 2:1), 35 grams of 30% by weight hydrogen peroxide and 1.15 grams of phosphoric acid (concentration is 85% by weight %) for mixing, start stirring, and react at 40°C and 0.015MPa, remove the water generated by the reaction in time during the reaction, and return the evaporated toluene to the reaction system for reuse, react for 5 hours, and perform gas chromatography Measure the content of m-HHP in the reaction solution to be 0.09% by weight, cool the reaction solution to 10°C for suction filtration, wash the obtained filter cake with a small amount of 10°C toluene and distilled water, dry the filter cake after washing, and obtain the p-DHP product , the yield was 93.1%, and the purity was 98.6%.
[0048] 2) The obtained filtrate removes the water phase to obtain an oil phase, and the oil phase is washed with a small amount of distilled water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com