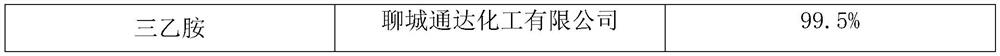Quality control method of loquat leaf lung-moistening cough-relieving paste
A quality control method, a technique for moistening the lung and relieving cough, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve the problems of speculation by lawbreakers, the simple detection method of lotus leaf moistening lung and cough ointment, and not being well suitable for clinical requirements, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Identification of Loquat Leaf in Loquat Leaf Runfei Zhike Ointment
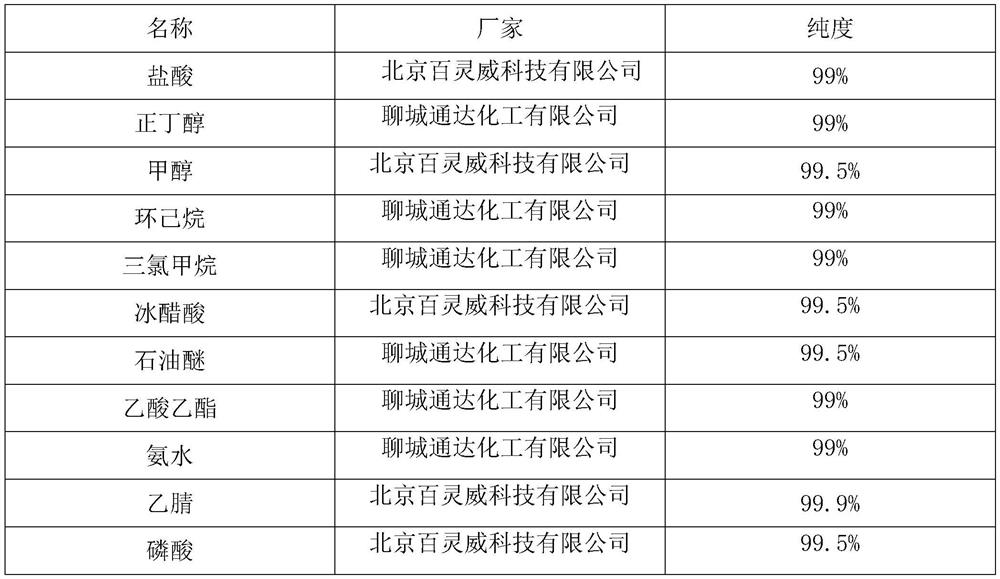
[0031] Take 20 mL of loquat leaf moistening lung and cough ointment, add 50 mL of hot water to it to dissolve, then add 2 mL of hydrochloric acid to it, shake well, and use it as the test solution; take 1.5 g of loquat leaf control medicinal material, add 50 mL of water, decoct for 1 h, Combine the filtrates, filter, put in a separatory funnel, add 2 mL of hydrochloric acid, shake well, then extract with 25 mL of n-butanol saturated with water, separate the n-butanol liquid, concentrate and evaporate to dryness, add 1 mL of methanol to dissolve the residue, and use it as a reference drug solution; According to the thin-layer chromatography test, draw 10 μL of the test solution and 10 μL of the reference medicinal material solution, and place them on the same silica gel G thin-layer plate respectively, with cyclohexane-chloroform-ethyl acetate-glacial acetic acid (20:5: 8:0.5) as developing a...
Embodiment 2
[0033] Example 2: Identification of Aster in Paye Runfei Zhike Ointment
[0034] Take 20 mL of Loquat Leaf Runfei Zhike Ointment, add 30 mL of petroleum ether at 60-90 ° C to it, heat and reflux for 30 minutes, filter, and the filtrate is concentrated to 1 mL as the test solution; Methane was 20mL, ultrasonically filtered for 15min, the filtrate was concentrated to 5mL, and used as the reference medicinal material solution; according to the thin-layer chromatography test, 10 μL of the test solution and 10 μL of the reference medicinal material solution were drawn, respectively, on the same silica gel G thin-layer plate, with 60- Petroleum ether-ethyl acetate (9:1) at 90°C was used as developing agent, developed, taken out, dried in the air, sprayed with dinitrophenylhydrazine solution, heated at 105°C for 10min, until the spots were clearly colored; Among them, at the position corresponding to the chromatogram of the control medicinal material, yellow spots of the same color a...
Embodiment 3
[0036] Example 3: Identification of Ephedra in Paye Runfei Zhike Ointment
[0037]Take 10 mL of Loquat Leaf Runfei Zhike Ointment, add 20 mL of methanol to it, ultrasonicate for 15 minutes, filter while hot and evaporate to dryness, add 4 mL of methanol to the residue to dissolve it completely, and use it as the test solution; The method is prepared as a reference medicinal solution; according to the thin-layer chromatography test, 10 μL of the test solution and 10 μL of the reference medicinal solution are drawn, and they are respectively spotted on the same silica gel G thin-layer plate, and mixed with n-butanol-methanol-chloroform-ammonia ( 8:1:3:1) as developing agent, develop, take out, dry, spray with ninhydrin solution, heat at 105°C for 5min, until the spots are clearly colored; At the position, the main spot of the same color appears under sunlight.
[0038] Conclusion: According to the above operation method, the test spots of Paye Runfei Zhike Cream are consistent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com