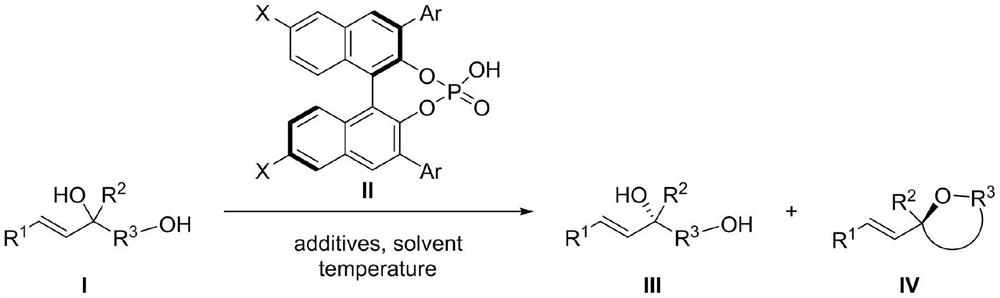
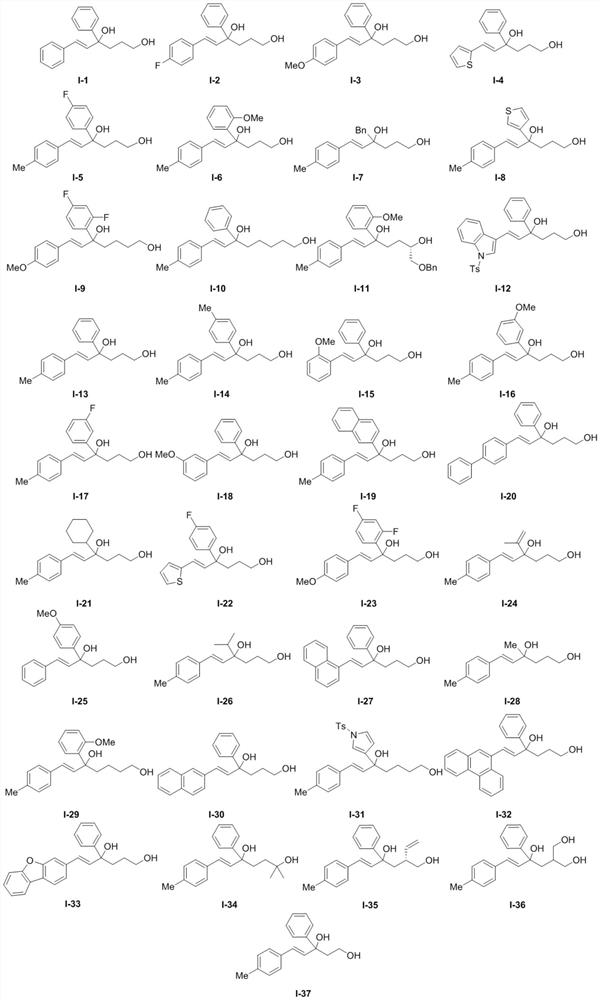
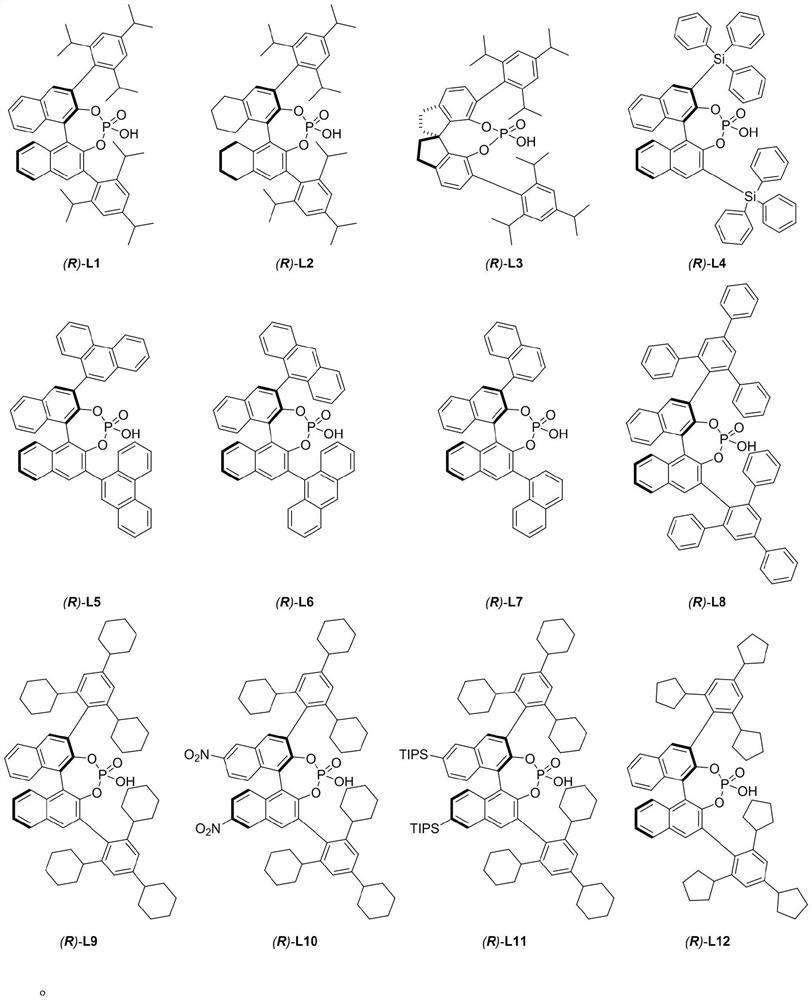
Chiral phosphoric acid catalyzed allyl tertiary alcohol kinetic resolution method
An allyl tertiary alcohol, kinetic resolution technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry and other directions, can solve the synthesis strategy difficult to promote tertiary alcohols, enantiomeric surface differentiation reduced, kinetics There are few problems such as splitting research, to achieve the effect of wide substrate application, high enantioselectivity and conversion rate, good application prospect and social value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of product III-1 and IV-1
[0029]
[0030] Experimental procedure: in N 2 Under protection, the reaction system was sealed for anhydrous and oxygen-free treatment, and the temperature was adjusted to 5°C. In a 25mL reaction flask, compound I-1 (0.05mmol, 1.0equiv) and chiral binaphthol catalyst (R) were sequentially added -L2 (0.0075mmol, 0.15equiv), additive (additive is: Molecular sieves (75mg)), and the solvent was 1,2-dichloroethane (5.0mL), and the reaction was carried out. The reaction was monitored by HPLC analysis until the reaction was completed, the reaction system was filtered through diatomaceous earth and concentrated, and the obtained concentrate was separated and purified to obtain target compound III-1 and target compound IV-1 respectively.
[0031] The resulting concentrated crude product was separated and purified by column chromatography (eluent: petroleum ether: ethyl acetate = 2:1 (v / v)) to obtain the target compou...
Embodiment 2
[0033] Embodiment 2: the synthesis of product III-2 and IV-2
[0034] The experimental method of embodiment 2 repeats embodiment 1, and difference is only " the compound shown in formula I-1 in embodiment 1 is replaced with the compound shown in formula I-2 of equivalent molar amount ", all the other operations The steps are the same as in Example 1 to finally obtain the corresponding compounds III-2 and IV-2.
[0035]
[0036] The product III-2 was obtained as a white solid with a yield of 42% and an ee value of 96%. [α] D 20 =–5.0 (c 1.0, CHCl 3 ). 1 H NMR (400MHz, DMSO–d 6 )δ7.53–7.47(m,2H),7.45–7.39(m,2H),7.38–7.27(m,4H),7.24–7.16(m,2H),6.63(d,J=16.0Hz,1H) ,6.58(d,J=16.1Hz,1H),5.32(s,1H),4.38(t,J=5.2Hz,1H),3.36–3.20(m,2H),1.98–1.77(m,2H), 1.98–1.80(m,1H),1.34–1.20(m,1H). 13 CNMR (100MHz, DMSO–d 6 )δ161.7(d, J=243.6Hz), 147.2, 137.5, 133.5(d, J=3.0Hz), 128.1(d, J=7.9Hz), 127.8, 126.1, 125.4, 124.7, 115.4(d, J =21.5Hz), 75.4, 61.2, 27.2. 19 F NMR (376MHz, DMSO–...
Embodiment 3
[0038] Embodiment 3: the synthesis of product III-3 and IV-3
[0039] The experimental method of embodiment 3 repeats embodiment 1, and difference is only " the compound shown in formula I-1 in embodiment 1 is replaced with the compound shown in formula I-3 of equivalent molar amount ", all the other operations The steps are the same as in Example 1 to finally obtain the corresponding compounds III-3 and IV-3.
[0040]
[0041] The product III-3 was obtained as a white solid with a yield of 42% and an ee value of 93%. [α] D 20 =–7.0 (c 1.0, CHCl 3 ). 1 H NMR (400MHz, Benzene-d 6 )δ7.56(dd,J=8.4,1.3Hz,2H),7.32–7.18(m,4H),7.16–7.00(m,1H),6.84–6.67(m,3H),6.37(d,J= 16.0Hz,1H),3.42–3.32(m,3H),3.28(s,3H),2.10–1.90(m,3H),1.65–1.32(m,2H),1.04–0.82(m,1H). 13 C NMR (100MHz, C 6 D. 6 )δ159.7,147.1,134.8,130.3,128.5,128.3,127.9,126.8,126.1,114.4,76.6,63.0,54.8,40.1,27.2.HRMS(ESI)m / z calcd.for C 19 h 22 NaO 3 [M+Na] + :321.1461; found 321.1460.
[0042] The product IV-3 wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com