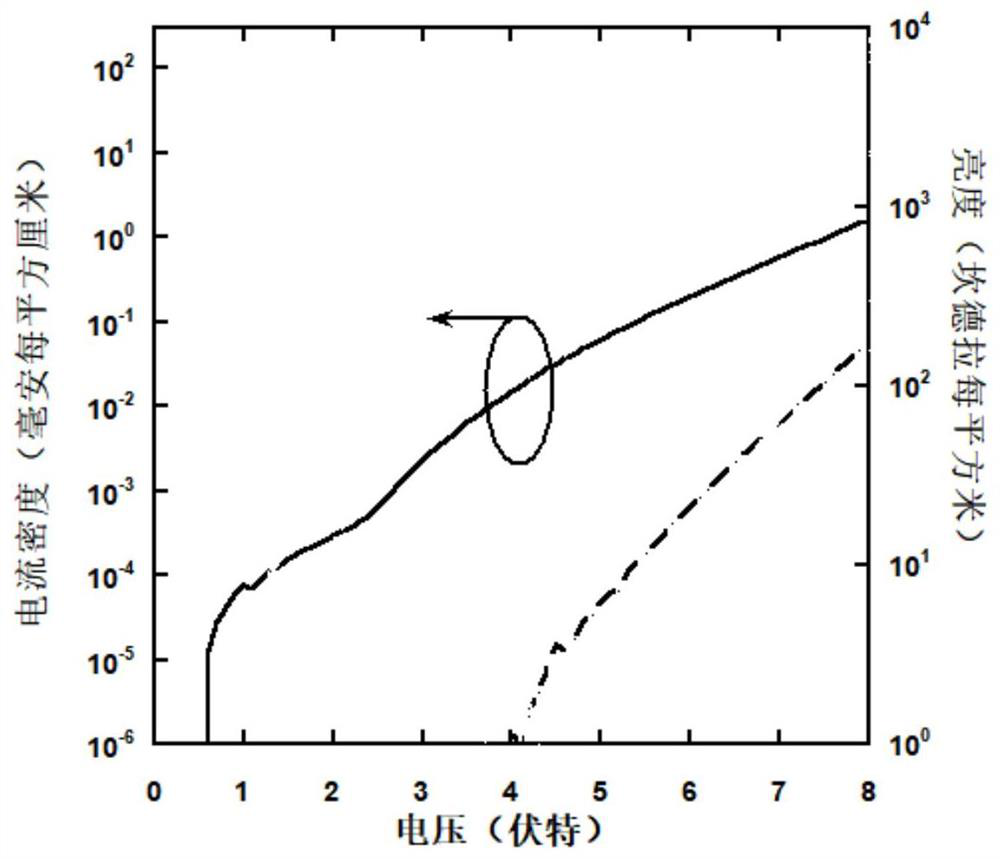
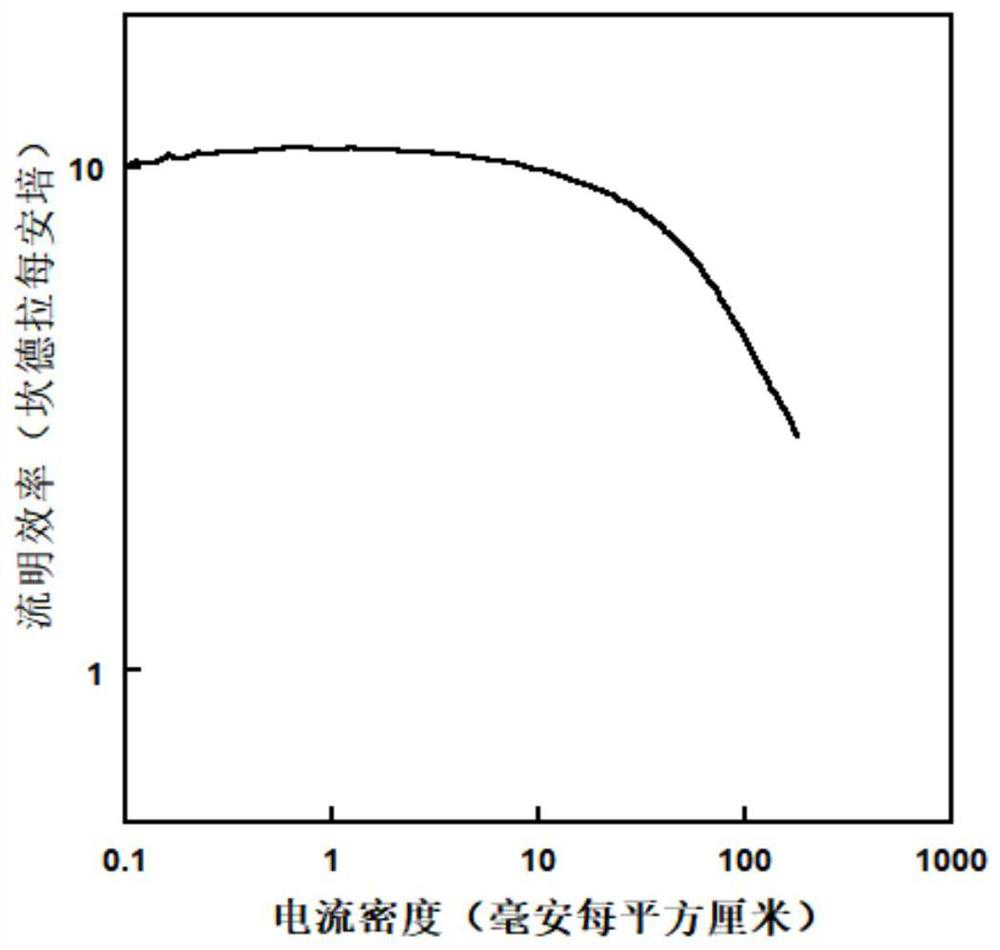
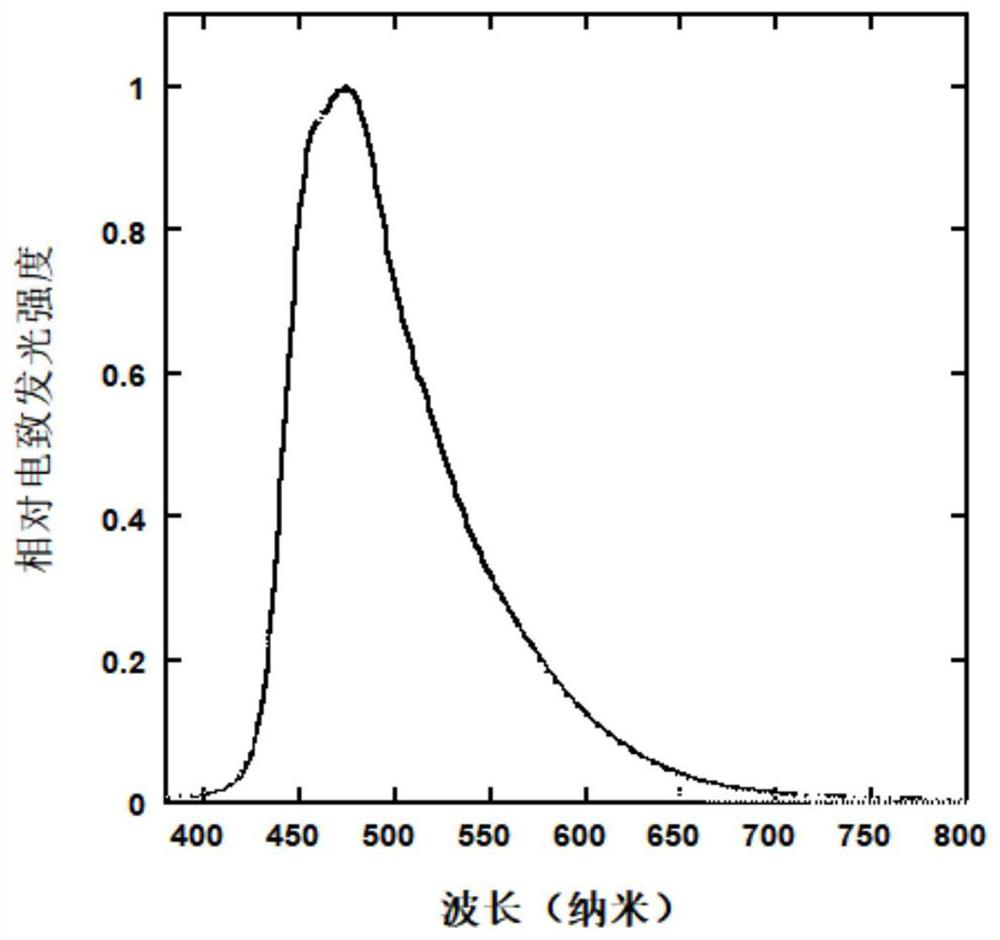
Organic small molecule hole transport material, preparation method and application thereof
A technology of hole transport materials and small molecules, which is applied in the field of organic small molecule hole transport materials and their preparation, can solve the problems such as the need to improve the luminous efficiency, and achieve the effects of excellent solubility, simple preparation method, and large steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of small molecules M1-3
[0042] (1) N,N-bis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)aniline (M1-1) synthesis:
[0043] Under argon atmosphere, 4,4-dibromotriphenylamine (1.00g, 2.48mmol), biboronic acid pinacol ester (1.89g, 7.44mmol), potassium acetate (1.46g, 14.89mmol), catalyst 1,1 '-bis(diphenylphosphino)ferrocenepalladium dichloride (Pd(dppf)Cl 2) (0.054g, 0.074mmol) was dissolved in 1,4-dioxane (20ml), heated to reflux, and reacted for 24h under reflux conditions; the solvent and potassium acetate were removed, purified by silica gel column chromatography, and the eluent Petroleum ether / dichloromethane=3 / 1 (volume ratio), and then recrystallized in tetrahydrofuran / ethanol to obtain a white powder (M1-1). 1 HNMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0044]
[0045] (2) N-phenyl-4-(9-phenyl-9H...
Embodiment 2
[0053] Preparation of Polymer M2-3
[0054] (1) 4-methyl-N, N-bis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)aniline ( Synthesis of M2-1):
[0055] Under argon atmosphere, 4-bromo-N-(4-bromophenyl)-N-(p-tolyl)aniline (1.00g, 2.40mmol), biboronic acid pinacol ester (1.83g, 7.19mmol), Potassium acetate (1.41 g, 14.38 mmol), catalyst 1,1'-bis(diphenylphosphino)ferrocenepalladium dichloride (Pd(dppf)Cl 2 ) (0.052g, 0.072mmol) was dissolved in 1,4-dioxane (20ml), heated to reflux, and reacted for 24h under reflux conditions; the solvent and potassium acetate were removed, purified by silica gel column chromatography, and the eluent Petroleum ether / dichloromethane=3 / 1 (volume ratio), and then recrystallized in tetrahydrofuran / ethanol to obtain a white powder (M2-1). 1 HNMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0056]
[0057] ...
Embodiment 3
[0064] Preparation of small molecule M3-3
[0065] (1) 4-methoxy-N,N-bis(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)aniline Synthesis of (M3-1):
[0066] Under argon atmosphere, 4-bromo-N-(4-bromophenyl)-N-(4-methoxyphenyl)aniline (1.00g, 2.31mmol), biboronic acid pinacol ester (1.76g, 6.93mmol), potassium acetate (1.36g, 13.86mmol), catalyst 1,1'-bis(diphenylphosphino)ferrocenepalladium dichloride (Pd(dppf)Cl 2 ) (0.051g, 0.069mmol) was dissolved in 1,4-dioxane (20ml), heated to reflux, and reacted for 24h under reflux conditions; the solvent and potassium acetate were removed, purified by silica gel column chromatography, and the eluent Petroleum ether / dichloromethane=3 / 1 (volume ratio), and then recrystallized in tetrahydrofuran / ethanol to obtain a white powder (M3-1). 1 HNMR, 13 CNMR, MS and elemental analysis results show that the obtained compound is the target product, and the chemical reaction equation of the preparation process is as follows:
[0067] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com