Application of salidroside derivative in external preparation for skin whitening
一种皮肤美白、外用剂的技术,应用在护肤品领域,能够解决破坏黑色素细胞、黑色素细胞不足等问题,达到增强美白活性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
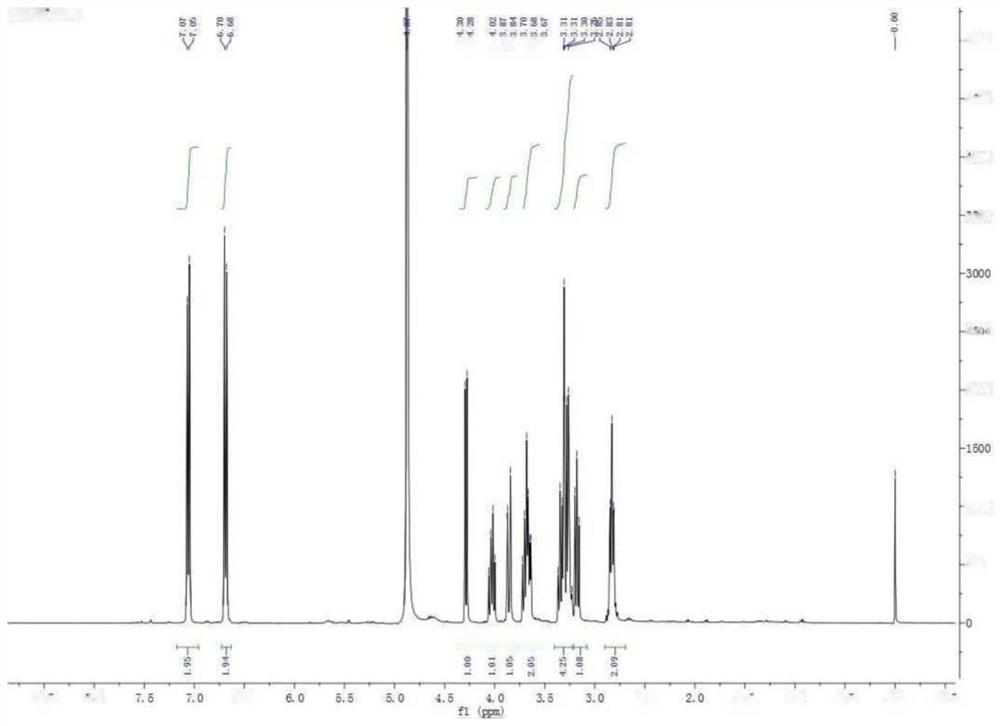
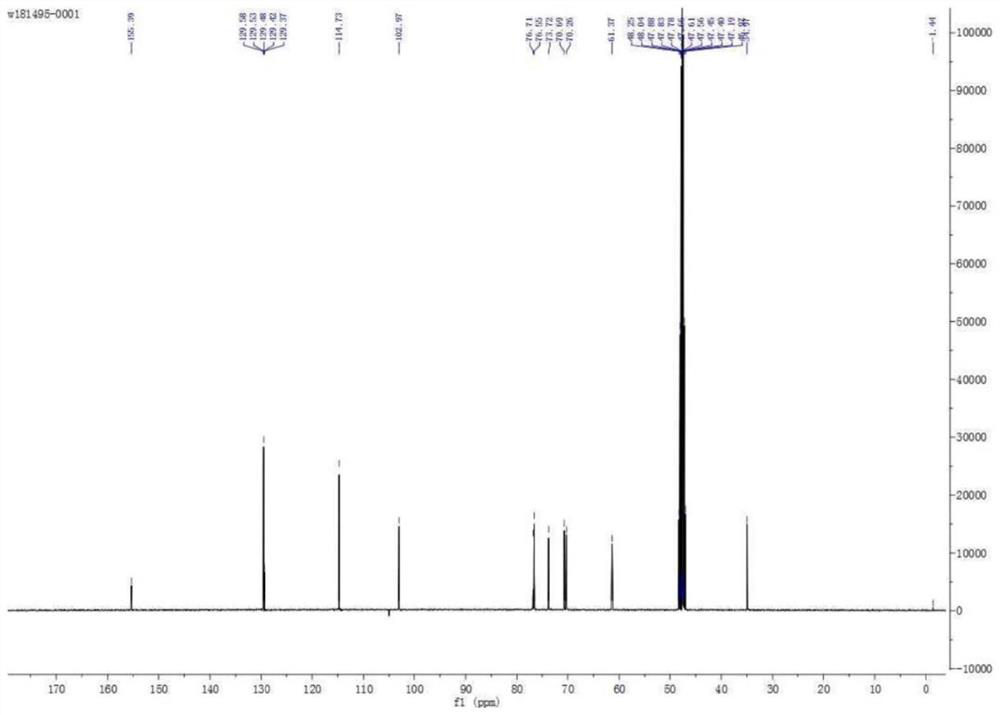
[0045] Example 1 : Preparation of 1-(3,5-dihydroxyphenyl)ethyl-β-D-glucoside
[0046]
[0047] step one:
[0048]
[0049] 1.18g of 3,5-dihydroxyphenylacetic acid and 8.4ml of BH3.THF (1.0M in THF) were dissolved in 20ml of THF and reacted at room temperature for 4 hours. After the reaction was completed, the solvent was removed by rotary evaporation to obtain a crude product. Purified by column chromatography to obtain 1.05 g of product with a yield of 97%.
[0050] Step two:
[0051]
[0052] Add 16.9g of intermediate 2, 6.9mL of benzyl chloride and 200mL of acetone in sequence in the reaction flask, stir to dissolve, add 8.3g of potassium carbonate and 0.8g of potassium iodide, and monitor the reflux reaction by TLC for 18h. The solvent was evaporated, the residue was poured into 200 mL of water, hydrochloric acid was added dropwise to adjust the pH to 7, and extracted with ethyl acetate. The organic phase was evaporated under reduced pressure and then recrystal...
Embodiment 2
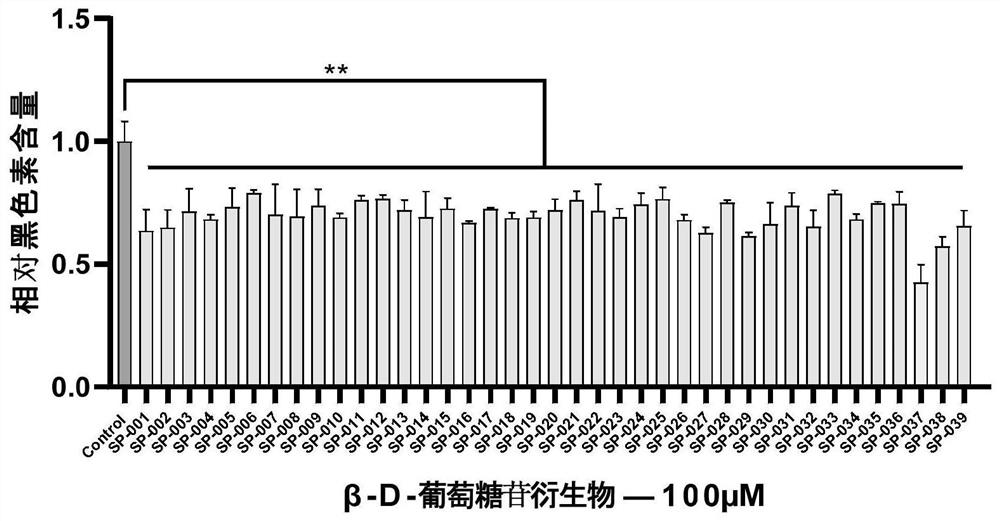
[0063] Example 2 : Effects of β-D-glucoside derivatives on melanin biosynthesis in melanocytes in vitro
[0064] a. Detection of activity of β-D-glucoside derivatives on inhibition of melanin production
[0065] Detection method:
[0066] In this part of the experiment, two kinds of tool cells, human melanocytes and instrumental melanocytes, were used for experiments. Human and instrumental melanocytes were seeded in 96-well plates with a plating density of 5000 cells / well. After the cells adhered to the wall, the culture medium was induced by adding nerve factors and induced by UV to simulate the production of melanin in vivo. Subsequently, the β-D-glucoside derivatives to be determined were added to the cells at a co-concentration of 100 μM to incubate the cells, and each group was set up with 3 replicates, and the cells were incubated at 37°C, 5% CO 2 The constant temperature and humidity incubator continued to cultivate for 60h. The cells were washed with pre-cooled p...
Embodiment 3
[0084] Example 3 : SP-037 Safety Evaluation
[0085] a. Mouse skin long-term toxicity test
[0086] experiment method:
[0087] In this part of the experiment, Kunming rats were used to carry out experiments. SP-037 was prepared with physiological saline, and the concentrations were 2.5% (mass volume ratio), 5% (mass volume ratio), and 10% (mass volume ratio). A control group, a 2.5% dose group, a 5% dose group and a 10% dose group were respectively set up. There was one mouse in the blank group, and two mice in each experimental group. Treatment with back skin smearing: first, use depilatory cream to remove the back hair of the mouse, apply the drug of corresponding concentration on the exposed part of the depilated skin every day, and administer the drug continuously for 3 months. During the experiment, take pictures regularly and record the weight of the mice, and observe Whether it can cause white spots, other skin irritation damage or poisoning.
[0088] Results and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com