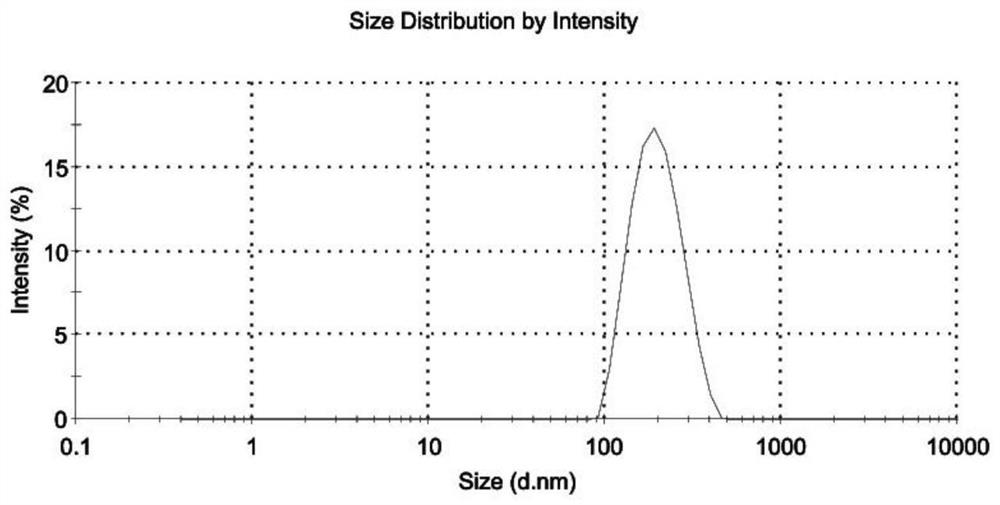
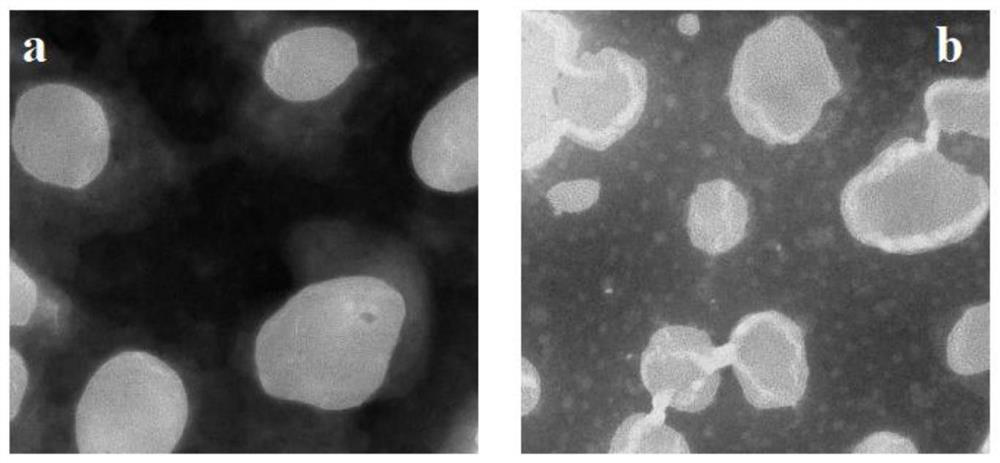
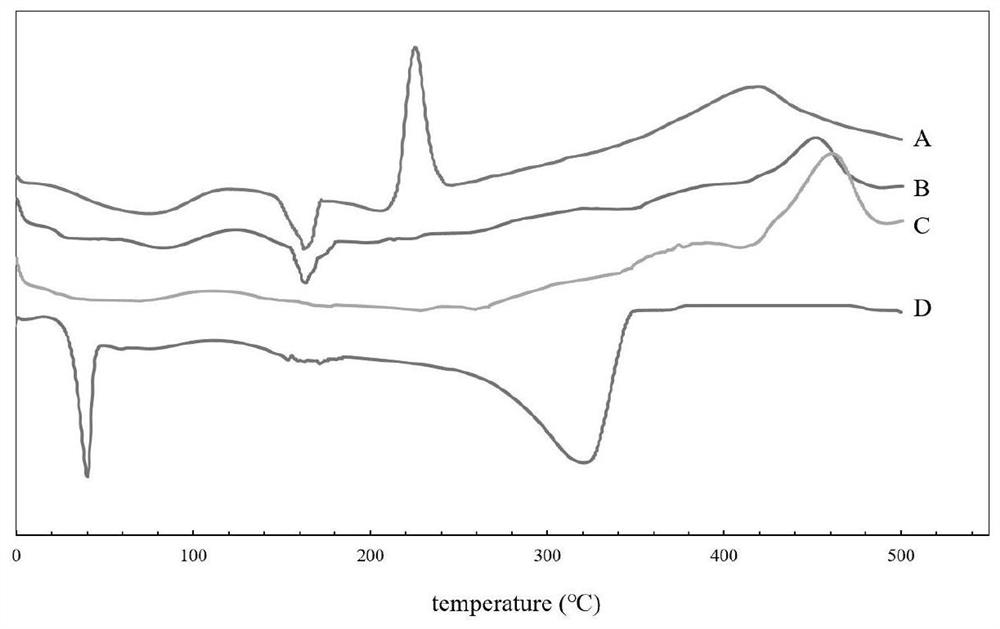
Albumin-coated cabazitaxel cationic nano-lipid carrier and preparation method thereof
The technology of a nano-lipid carrier and cabazitaxel is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve the problems of large dosage, large toxic and side effects, and potential safety hazards, etc. problems, achieve high encapsulation efficiency, uniform particle size, and improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Albumin-coated cationic nano-lipid carrier of cabazitaxel, including the following raw materials: 10 mg of cabazitaxel; 57 mg of stearic acid; 43 mg of glyceryl caprylate; 100 mg of egg yolk lecithin; 400 mg of Brij20; ; Human serum albumin 40mg.
[0037] Its preparation process is as follows:
[0038] Dissolve cabazitaxel, stearic acid, glyceryl caprylate, and egg yolk lecithin in an organic solvent at about 75°C (acetone:ethanol=1:1, 4ml in total), and use it as the oil phase for later use; mix Brij20 and hexadecyl Dissolve trimethylammonium bromide in ultrapure water at about 75°C, and use it as the water phase for later use; at a stirring speed of 1000r / min, use a syringe to absorb the oil phase and inject it into the water phase at a certain speed, and continue stirring and emulsifying at 75°C 1h, while hot, slowly drop the emulsion into pre-cooled ultrapure water, and continue stirring for 1h in an ice-bath environment to obtain Cbz-cNLCs. Take a certain amount ...
Embodiment 2
[0041] Albumin-coated cationic nano lipid carrier of cabazitaxel, including the following raw materials: cabazitaxel 10mg; stearic acid 57mg; caprylic / capric glycerides 43mg; egg yolk lecithin 100mg; Brij20 100mg; Ammonium chloride 10mg; human serum albumin 40mg.
[0042] Its preparation process is as follows:
[0043]Dissolve cabazitaxel, stearic acid, glyceryl caprate, and egg yolk lecithin in an organic solvent at about 75°C (acetone:ethanol=1:1, 4ml in total), and use it as the oil phase for later use; mix Brij20 and hexadecane Dissolve trimethylammonium bromide in ultrapure water at about 75°C, and use it as the water phase for later use; at a stirring speed of 1000r / min, use a syringe to absorb the oil phase and inject it into the water phase at a certain speed, and continue stirring at 75°C After emulsifying for 1 hour, slowly drop the emulsion into pre-cooled ultrapure water while it is still hot, and continue stirring for 1 hour in an ice-bath environment to obtain C...
Embodiment 3
[0046] Albumin-coated cationic nano lipid carrier of cabazitaxel, including the following raw materials: cabazitaxel 10mg; stearic acid 57mg; caprylic / capric glycerides 43mg; egg yolk lecithin 200mg; Ammonium chloride 10mg; human serum albumin 40mg.
[0047] Its preparation process is as follows:
[0048] Dissolve cabazitaxel, stearic acid, caprylic / capric glyceride, egg yolk lecithin in an organic solvent (acetone:ethanol=1:1, 4ml in total) at about 75°C, and use it as the oil phase for later use; Brij20 and ten Hexaalkyltrimethylammonium bromide is dissolved in ultrapure water at about 75°C and used as the water phase for later use; at a stirring speed of 1000r / min, use a syringe to absorb the oil phase and inject it into the water phase at a certain speed, at 75°C, Continue stirring and emulsifying for 1 hour, slowly drop the emulsion into pre-cooled ultrapure water while it is hot, and continue stirring for 1 hour in an ice-bath environment to obtain Cbz-cNLCs. Take a ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com