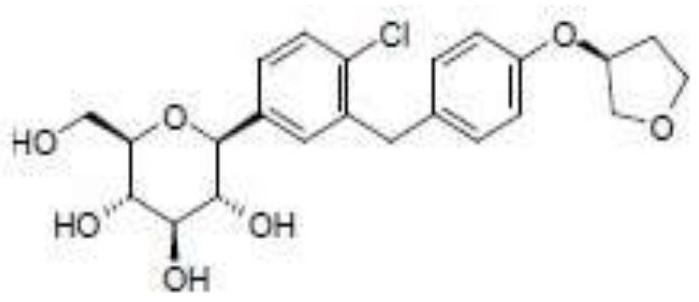
A kind of Empagliflozin medicinal composition and preparation method thereof
A technology of empagliflozin and its composition, which is applied in the field of empagliflozin pharmaceutical composition and its preparation, can solve the problems of increased risk of hypoglycemia, β cell toxicity, weight gain, etc., and achieve improved blood sugar control, The effect of prolonging the action time and reducing the fluctuation of blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of the pharmaceutical composition containing empagliflozin and saxagliptin, the steps are as follows:
[0032] ① Weigh all saxagliptin, half of empagliflozin, filler, 1 / 2-2 / 3 disintegrant, skeleton material, sieve, and mix well;
[0033] ② Prepare binder solution, wet granulate, dry at 60°C, and granulate with 40 mesh sieve;
[0034] ③ Add the remaining empagliflozin, the remaining disintegrant croscarmellose sodium, glidant, and lubricant, mix well and press into tablets, compound tablets.
[0035] The specifications of the obtained tablet are respectively: saxagliptin 1-15mg; empagliflozin 0.5-30mg.
Embodiment 4
[0037] Experimental group: the compound tablet prepared in Preparation Example 1 of the present invention. Batch number: 20191113.
[0038] Control group: 1. Saxagliptin tablets; specification: 5 mg; manufacturer: AstraZeneca Pharmaceutical Co., Ltd.; 2. Empagliflozin tablets; specification: 10 mg; manufacturer: Shanghai Boehringer Ingelheim Pharmaceutical Co., Ltd.
[0039] Instrument: Waters ARC high performance liquid chromatography, including: pump system, diode array detector and workstation.
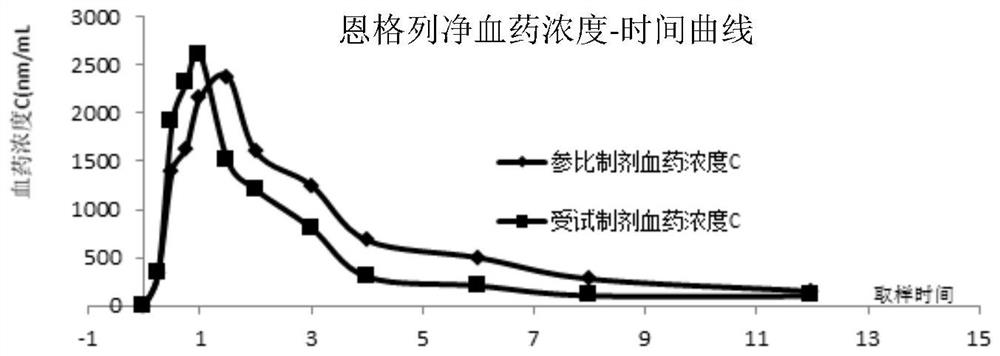
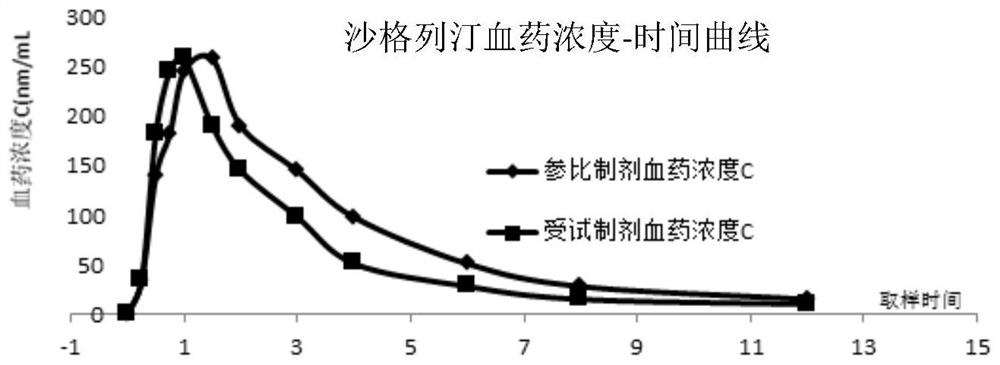
[0040] Test plan: select 6 Beagle dogs, half male and half male, average body weight 10.0 ± 1.0Kg, be divided into 3 groups at random, be respectively control group (saxagliptin), control group (empagliflozin), treatment group (implementation). Example 1 sample). Fasting 8h before the administration experiment, blank blood was drawn before the administration, the experimental group took 1 compound tablet prepared in Example 1, and the control group took the reference preparation Sa...
Embodiment 5
[0044] The administration method and group setting are the same as in Example 4, and the hypoglycemic effect of the sample in Example 1 of the present invention in the human body is investigated.
[0045] Dosing regimen: 12 patients with type Ⅱ diabetes mellitus were randomly divided into treatment group, control group 1, and control group 2, each with 4 cases. Among them, the treatment group was given the test preparation, and the control group was given the reference preparation, once a day. The drug was administered for 8 consecutive weeks, and fasting blood glucose (FPG), two-hour postprandial blood glucose (2hPG) and glycosylated hemoglobin (HbA1c) were detected before and after treatment at 0, 4, and 8 weeks. The experimental results are shown in the table below:
[0046]
[0047] The experimental results show that compared with the reference preparation, using the compound saxagliptin / empagliflozin tablet provided by the preparation embodiment 1 of the present invent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com