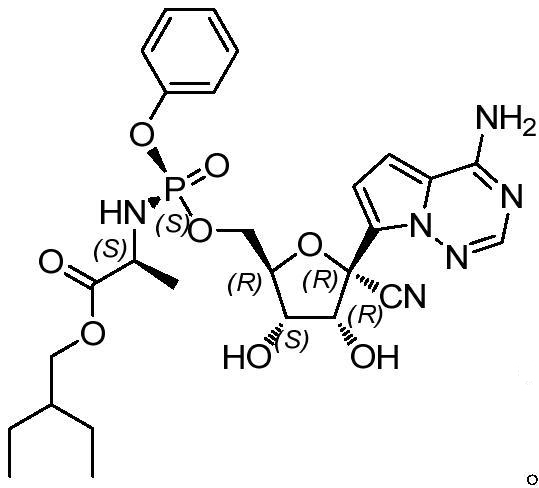
Method for detecting related substances of retegravir intermediate
A detection method and technology for related substances, which are applied in the field of detection of related substances in intermediates of remdesivir, can solve the problems that there is no detection method for related substances in intermediates, and achieve the effects of stability, strong adaptability, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
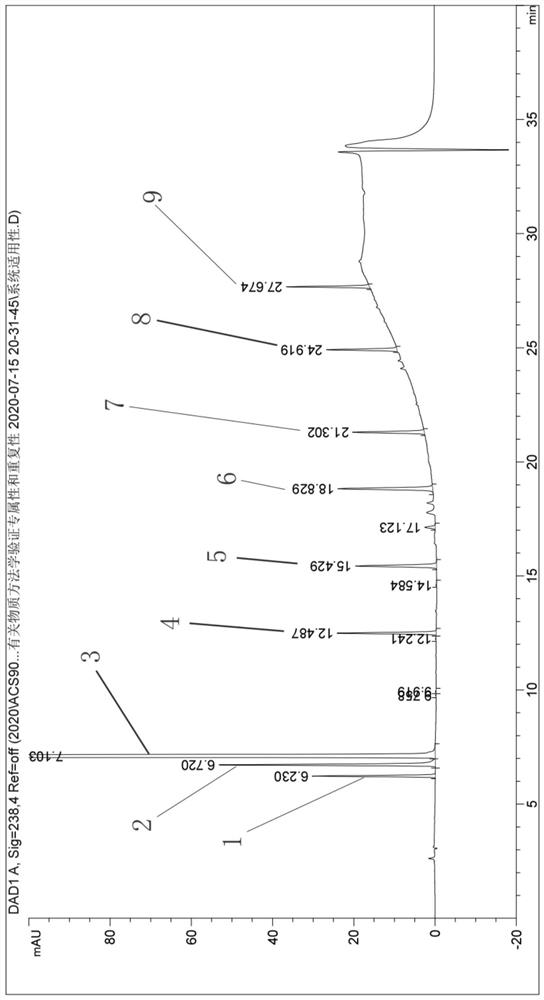
[0039] Embodiment 1: specificity test
[0040] 1. Detection of chromatographic conditions
[0041] Chromatographic column: C18 (250mm×4.6mm, 5μm);
[0042] Detector: Diode Array Detector (DAD);
[0043] Detection wavelength: 238nm;
[0044] Flow rate: 1.0ml / min;
[0045] Column temperature: 30°C;
[0046] Diluent: 0.1% phosphoric acid water-methanol (95:5);
[0047] Injection volume: 5 μl.
[0048] 2. Preparation of reference substance and test solution
[0049] The specificity test needs to verify that the blank solution has no interference at the retention time of the main peak in the test product and the reference solution, and the separation between impurities and main components.
[0050] The following is the preparation method of each impurity and main component:
[0051]Impurity a positioning solution: Accurately weigh 15 mg of impurity a reference substance, put in a 50 ml measuring bottle, add 5 ml of methanol first, then add 0.25 ml of 85% phosphoric acid, di...
Embodiment 2
[0067] Embodiment 2: sensitivity test
[0068] Take the system suitability solution prepared in Example 1 and dilute it step by step to an appropriate multiple. The solution with a signal-to-noise ratio ≥ 10: 1 is used as the solution for the limit of quantification; the solution with a signal-to-noise ratio ≥ 3: 1 is used as the solution for the limit of detection.
[0069] With reference to the detection chromatographic conditions of Example 1, accurately measure 5 μl of each of the above solutions, inject them into a liquid chromatograph, inject 6 injections of the quantification limit solution continuously, inject 1 injection of the detection limit solution, and record the chromatogram. The results are shown in Table 2~ table 3.
[0070] Table 2 Quantitative limit verification results
[0071] name Concentrationμg / ml S / N Sensitivity (%) Impurity a 0.11708 24.68 0.05 Impurity b 0.11746 38.48 0.05 intermediate 0.12240 21.07 0.05 ...
Embodiment 3
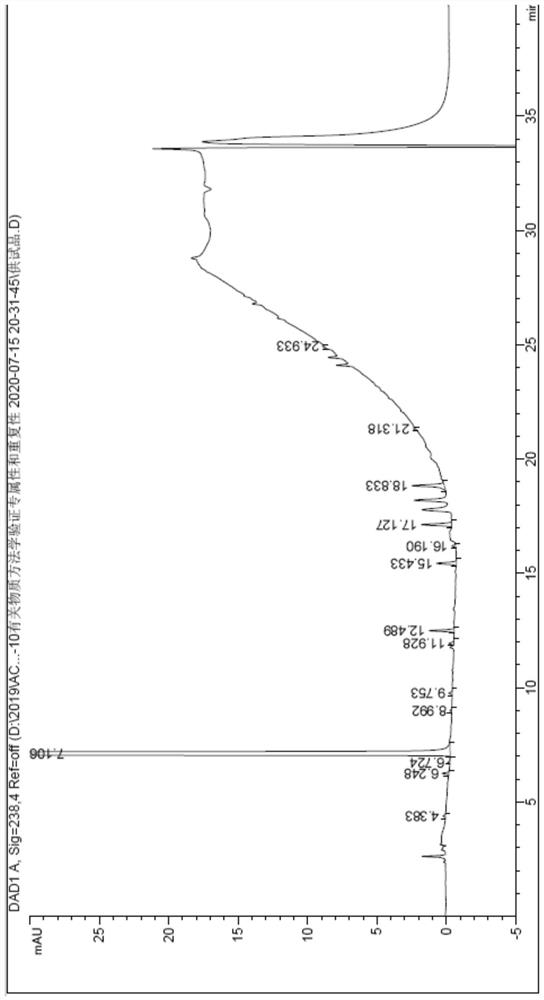
[0075] Embodiment 3: repeatability test
[0076] The test solution: Weigh about 12.5mg of the test product, put it in a 50ml measuring bottle, first add 5ml of methanol, then add 0.25ml of 85% phosphoric acid, dissolve it by ultrasonic, dilute with mobile phase A to the volume, shake well, Prepare 6 copies in parallel.
[0077] With reference to the detection chromatographic conditions of Example 1, 5 μl of each of the above solutions were precisely measured, injected into a liquid chromatograph, and the chromatogram was recorded. The results are shown in Table 4.
[0078] Table 4 Repeatability inspection results
[0079]
[0080]
[0081] Note: "ND" means not detected.
[0082] As can be seen from Table 4, the number of impurity peaks above the limit of quantification remains unchanged, and the RSDs of single impurity content and purity all meet the requirements (the RSD of each impurity with a content of less than 0.5% is not more than 10.0%; Each impurity RSD is no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com