Preparation method of Relugolix and intermediate compounds
A compound, a technology of hydrazine hydrate, applied in the field of preparation of Relugolix, can solve problems such as being unsuitable for practical production and application, difficult to remove, etc., and achieve the effects of good industrial application prospect, less impurities and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
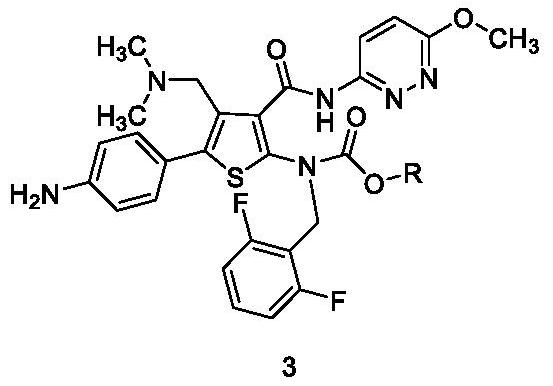
[0041] Embodiment 1: the preparation of compound 3-a
[0042]
[0043] Compound 2-a (25.0 g) was put into methanol (200.0 ml), 10% Pd / C (2.5 g) and ammonium formate (4.77 g) were added thereto, and stirred at 50±5° C. for 4 hours. After the reaction was completed, the mother liquor was collected by filtration, and the filter cake was washed with methanol. Raise the mother liquor to 40±5°C and stir for 1 hour, then cool down to 5±5°C, collect crystals by filtration, and wash the filter cake with cold methanol. Drying under reduced pressure gave compound 3-a (22.0 g). yield: 92%, HPLC purity 99.64%.
[0044] 1 H NMR (400MHz, Chloroform-d) δ14.06(d, J=27.0Hz, 1H), 8.56(d, J=9.6Hz, 1H), 7.66(s, 1H), 7.54(d, J=8.1Hz ,2H),7.24(d,J=8.1Hz,2H),7.16–7.07(m,1H),6.98(d,J=9.5Hz,1H),6.73(t,J=7.7Hz,2H),5.03 (s,2H),4.23(s,1H),4.10(s,4H),3.82(s,3H),3.49(s,2H),2.19(s,6H),1.77(s,1H),1.64( s,2H),1.01(s,1H),0.79(t,J=7.1Hz,2H).
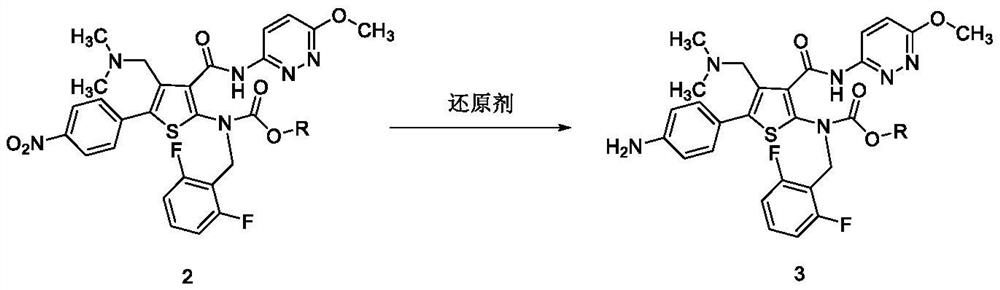
Embodiment 2
[0045] Embodiment 2: the preparation of compound 4-a
[0046]
[0047] Compound 3-a (10.0g) and 1,1'-carbonyldiimidazole (5.31g) were put into dichloromethane (40.0ml), and N,N-diisopropylethylamine (4.24g ), and stirred at 20±5° C. for 1-2 hours (hereinafter referred to as reaction mixture A).
[0048]Methoxylamine hydrochloride (13.6eq) was put into dichloromethane (60.0ml), and N,N-diisopropylethylamine (21.18g) was added to the mixture, and stirred at 25±5°C for 1 hour (hereinafter referred to as reaction mixture B). Reaction mixture B was slowly added to reaction mixture A at 0±5°C, and stirred at the same temperature for 1-2 hours. At 0±5°C, add saturated sodium bicarbonate solution to the reaction mixture, stir for 30 minutes and let it stand, and separate the organic phase, wash with saturated sodium bicarbonate solution, distill off the organic solvent under reduced pressure, and wash the residue with ethyl acetate Recrystallized from isopropyl ether to obtain c...
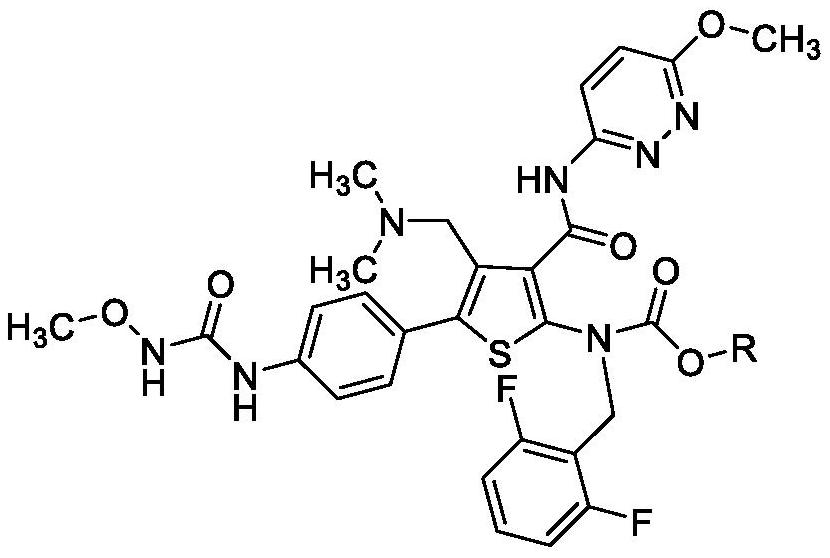
Embodiment 3
[0050] Embodiment 3: preparation compound 1
[0051]
[0052] Compound 4-a (5.0 g) was added to methanol (50.0 ml), sodium methoxide (0.59 g) was added to the mixture, and stirred at room temperature for 2-3 hours. Cool to 0°C or lower and stir for 2-4 hours, collect crystals by filtration, wash the filter cake with methanol and dry under reduced pressure at 50-60°C. The obtained solid was put into tetrahydrofuran, the temperature of the mixture was raised to 60±5°C, and stirred at the same temperature for 1 hour, the mixture was cooled to 0°C, and aged with stirring at the same temperature for 2 hours. The crystals were collected by filtration, and the filter cake was washed with tetrahydrofuran. The filter cake was recrystallized from dimethyl sulfoxide (DMSO) and ethanol to obtain compound 1 (4.25 g). Yield: 93%, HPLC purity 99.68%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com