Synthesis method of henatinib intermediate and obtained henatinib intermediate
A synthetic method and intermediate technology, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of environmental protection, high cost, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
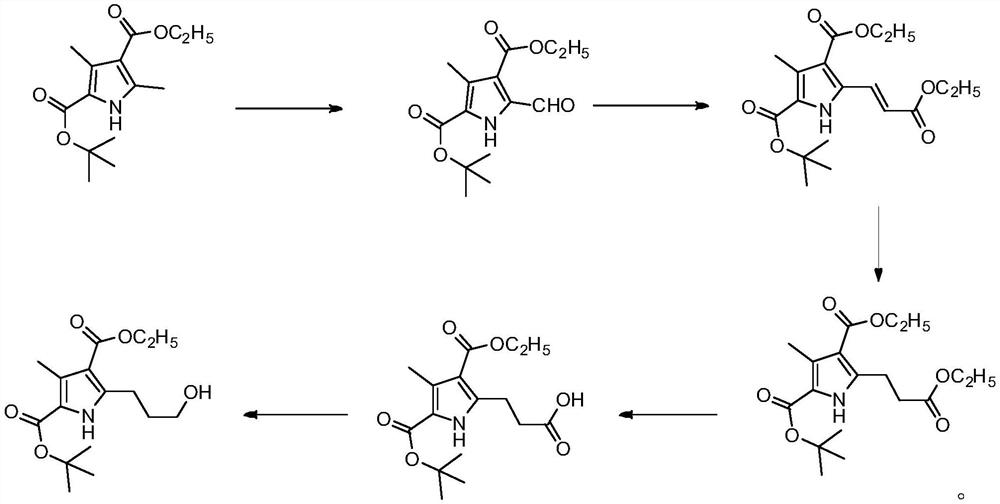
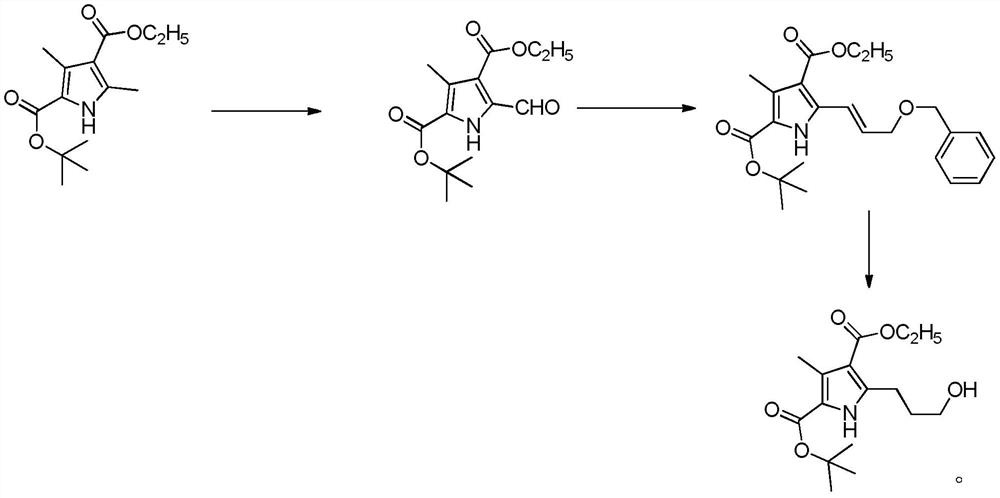
[0027] A kind of synthetic method of henatinib intermediate of the present invention, comprises the following steps:
[0028] 1) Take 3,5-dimethyl-1H-pyrrole-2,4-dicarboxylic acid-2-tert-butyl-4-ethyl ester as a starting material, add it to an organic solvent, stir at room temperature, and make it Dissolve, then add cerium ammonium nitrate, the ratio of the amount of cerium ammonium nitrate to the starting material is 3:1-4:1, stir at room temperature, fully react until the end of the reaction, purify, and obtain intermediate 1;
[0029] 2) Under the protection of an inert gas, add intermediate 1 to an organic solvent, stir at room temperature to dissolve it, and then add (benzyloxyethyl)phenylphosphine, (benzyloxyethyl)phenylphosphine The ratio of the amount of substances to the intermediate 1 is 1.2:1-1.5:1, stirred at room temperature, fully reacted, and purified to the end of the reaction to obtain intermediate 2;
Embodiment 1
[0041] A kind of synthetic method of henatinib intermediate of the present invention, comprises the following steps:
[0042] 1) Preparation of formyl-3-methyl-1H-pyrrole-2,4-dicarboxylic acid 2-tert-butyl ester 4-ethyl ester (intermediate 1)
[0043] In a 2L reaction flask, add 590g of tetrahydrofuran and 720g of acetic acid, under stirring at room temperature, add 60g of 2-tert-butyl 4-ethyl 3,5-dimethyl-1H-pyrrole-2,4-dicarboxylate (0.22mol ) as the starting material, stirred and dissolved, then added cerium ammonium nitrate 437g (0.8mol) to obtain the mixture; the mixture was stirred at room temperature for 0.5h, and TLC was monitored until the reaction was complete. The TLC detection conditions were: silica gel GF254 thin-layer plate, developer It is a mixture of n-hexane and ethyl acetate with a volume ratio of 8:1, the main spot Rf is 0.5, the raw material Rf is 0.4, and the color is developed by ultraviolet light;
[0044] Under the condition of stirring, the reaction...
Embodiment 2
[0057] A kind of synthetic method of henatinib intermediate of the present invention, comprises the following steps:
[0058] 1) Preparation of formyl-3-methyl-1H-pyrrole-2,4-dicarboxylic acid 2-tert-butyl ester 4-ethyl ester (intermediate 1)
[0059] In a 2L reaction flask, add 590g of tetrahydrofuran and 720g of acetic acid, and add 2-tert-butyl 4-ethyl 3,5-dimethyl-1H-pyrrole-2,4-dicarboxylate under stirring at room temperature (23°C) 60g (0.22mol) was used as the starting material, stirred and dissolved, and then 361g (0.66mol) of ammonium cerium nitrate was added to obtain a mixture; the mixture was stirred at room temperature for 1.0h, and TLC was monitored until the reaction was complete. The TLC detection conditions were: silica gel GF254 thin For laminates, the developer is a mixture of n-hexane and ethyl acetate with a volume ratio of 8:1, the main spot Rf is 0.5, the raw material Rf is 0.4, and the ultraviolet color is developed;
[0060] Under the condition of sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com