Synthesis method of boron-nitrogen benzocarbazole derivative
A technology of benzocarbazole and synthesis method, which is applied in the field of synthesis of borazine benzocarbazole derivatives, can solve the problems of cumbersome post-processing, harsh reaction conditions, and difficulty in realizing the synthesis of boron-nitrogen skeletons with novel structures.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
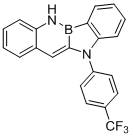
[0028] Synthetic compound:
[0029]
[0030] Take a 2L dry three-necked flask, evacuate and change argon for 3 times, under the protection of nitrogen, add o-aminostyrene (14.4g, 0.12mol), potassium phenyl-o-bromofluoroborate (26.2g, 0.1mol), four Silicon chloride (17.0g 0.1mol), aniline (9.5g, 0.12mol), bis(dibenzylideneacetone)palladium (575mg, 1mol%), 2-dicyclohexylphosphine-2',4',6' - Triisopropylbiphenyl (952 mg, 2mol%), sodium tert-butoxide (11.5g, 0.12mol), and anhydrous and oxygen-free solvent toluene (500mL) were introduced. Under the protection of argon, the reaction was carried out at 100°C for about 8 hours. After the reaction was completed, after the temperature of the system dropped to room temperature, ethyl acetate (4*1L) and water (2L) were extracted, dried, and recrystallized to obtain 25.6 g of a white solid with a yield of 87 %.
[0031] The nuclear magnetic analysis data of this compound are as follows:
[0032] 1 H NMR (400 MHz, CDCl 3 ): δ 8.63 (...
Embodiment 2
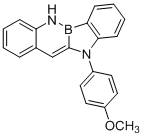
[0034] Synthetic compound:
[0035]
[0036]Take a 2L dry three-necked flask, evacuate and change argon for 3 times, under the protection of nitrogen, add o-aminostyrene (14.4g, 0.12mol), potassium phenyl-o-bromofluoroborate (26.2g, 0.1mol), four Silicon chloride (17.0g 0.1mol), p-methoxyaniline (14.8g, 0.12mol), bis(dibenzylideneacetone) palladium (290mg, 0.5mol%), 2-dicyclohexylphosphine-2', 4',6'-Triisopropylbiphenyl (480 mg, 1mol%) and sodium tert-butoxide (11.5 g, 0.12mol) were introduced into anhydrous and oxygen-free solvent toluene (500mL). Under the protection of argon, react at 80°C for about 4 hours. After the reaction is completed, after the temperature of the system drops to room temperature, extract with ethyl acetate (4*1L) and water (2L), combine the organic phases, dry them with anhydrous sodium sulfate, and recrystallize 26.9 g of white solid was obtained, with a yield of 83%.
[0037] The nuclear magnetic analysis data of this compound are as follows: ...
Embodiment 3
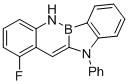
[0040] Synthetic compound:
[0041]
[0042] Take a 2L dry three-necked flask, vacuumize and change the argon for 3 times, under the protection of nitrogen, add o-aminostyrene (14.4 g, 0.12mol), potassium phenyl-o-bromofluoroborate (26.2g, 0.1mol), four Silicon chloride (17.0g 0.1mol), p-trifluoromethylaniline (19.4g, 0.12mol), allyl palladium(II) chloride dimer (183mg, 0.5mol%), 2-(di-tert-butyl Phosphine)biphenyl (298 mg, 1mol%), sodium tert-butoxide (11.5g, 0.12mol), and anhydrous and oxygen-free solvent tetrahydrofuran (500mL). Under the protection of argon, react at 60°C for about 8 h. After the reaction is completed, after the temperature of the system drops to room temperature, extract with ethyl acetate (4*1L) and water (2L), combine the organic phases, dry over anhydrous sodium sulfate, and re- Crystallized to obtain 28.2 g of white solid, yield 78%.
[0043] The nuclear magnetic analysis data of this compound are as follows:
[0044] 1 H NMR (400 MHz, CDCl 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com