Synthetic method of androstenone
A synthetic method and technology of androstenone, applied in the field of pharmaceutical preparation, can solve the problems of industrial production limitation and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
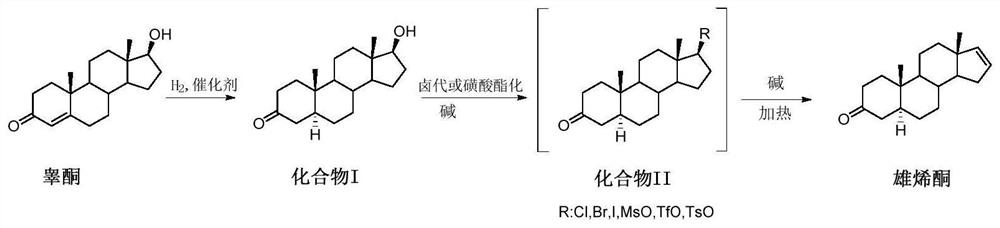
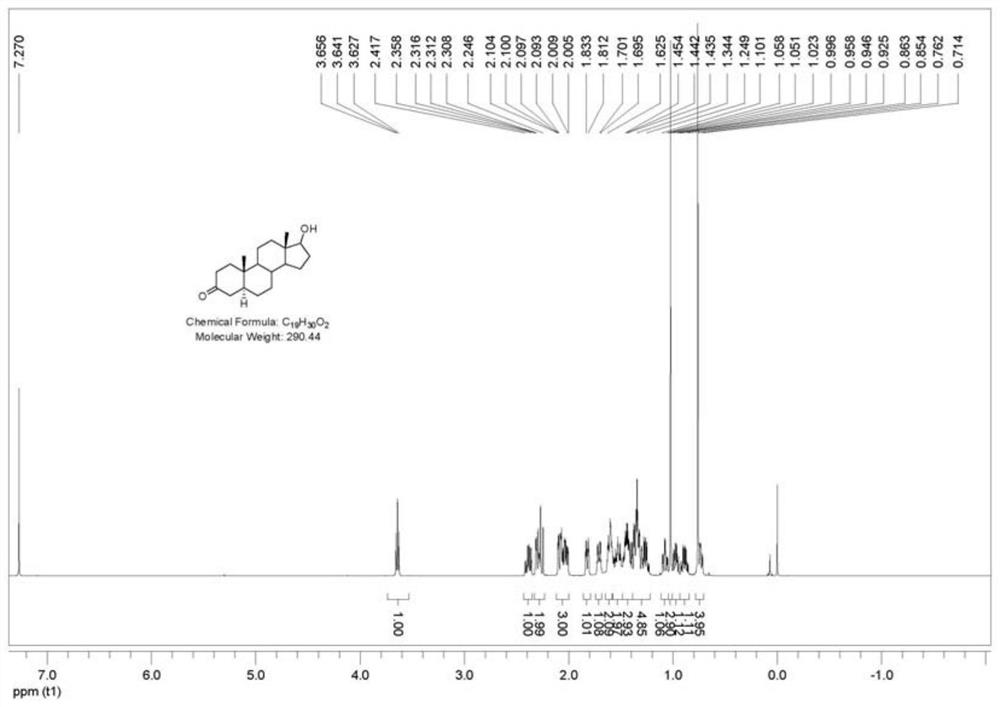
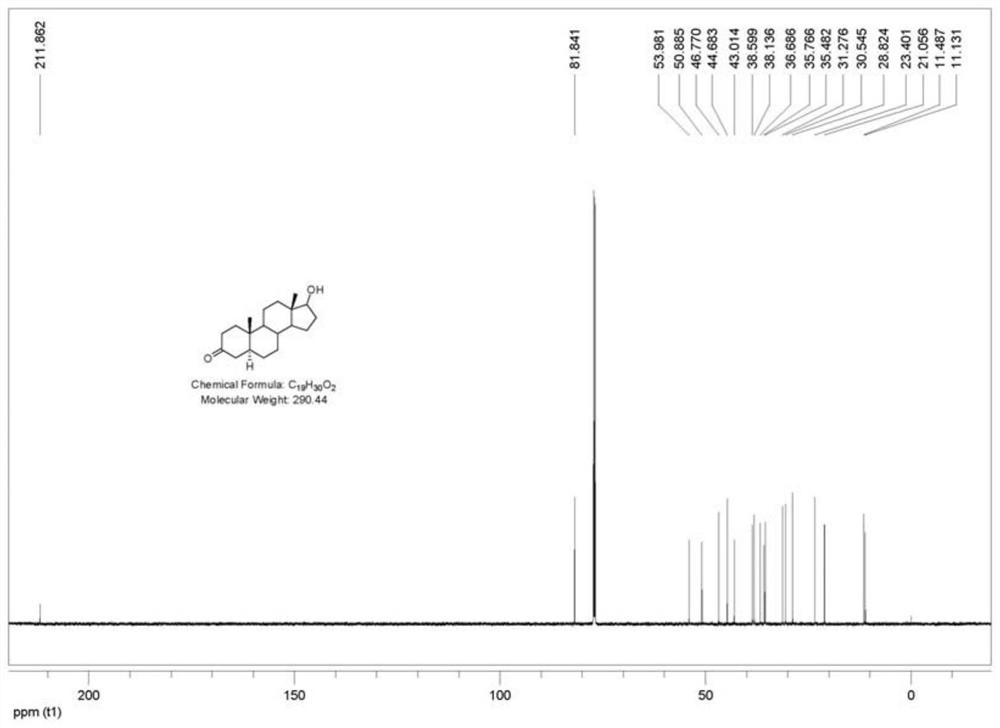
[0088] 1. Preparation of Compound I
[0089] In the autoclave of 10L, add testosterone respectively, namely testosterone (1000g, 3.472mol), methanol (5000mL, 5v / w) and 50g palladium carbon (50% water content, 5% palladium content), nitrogen replacement protection, then Start stirring and cool down to ≤-20°C, then replace nitrogen with hydrogen, keep the pressure in the kettle at 0.2MPa, and react at constant temperature and pressure for 8 hours. After the reaction is monitored by TLC, nitrogen is used for three times, and then the material is withdrawn and recovered by filtration The catalyst and filter cake were washed with methanol, the combined filtrates were concentrated under reduced pressure to recover methanol, and then vacuum-dried to ensure that the methanol content was less than 1% to obtain compound I crude product, white solid (1000 g, crude product yield 99%).
[0090] 2. Preparation of the target product androstenone
[0091] Compound I (500g, 1.724mol) and trie...
Embodiment 2
[0098] 1. Preparation of Compound I
[0099] In the autoclave of 10L, add testosterone respectively, namely testosterone (1000g, 3.472mol), methanol (5000mL, 5v / w) and 50g palladium carbon (50% water content, 5% palladium content), nitrogen replacement protection, then Start stirring and cool down to ≤-20°C, then replace nitrogen with hydrogen, keep the pressure in the kettle at 0.2MPa, and react at constant temperature and pressure for 8 hours. After the reaction is monitored by TLC, nitrogen is used for three times, and then the material is withdrawn and recovered by filtration The catalyst and filter cake were washed with methanol, the combined filtrates were concentrated under reduced pressure to recover methanol, and then vacuum-dried to ensure that the methanol content was less than 1% to obtain compound I crude product, white solid (1000 g, crude product yield 99%).
[0100] 2. Preparation of the target product androstenone
[0101] Compound I (500g, 1.724mol) and trie...
Embodiment 3
[0103] 1. Preparation of Compound I
[0104] In the autoclave of 10L, add testosterone respectively, namely testosterone (1000g, 3.472mol), methanol (5000mL, 5v / w) and 50g palladium carbon (50% water content, 5% palladium content), nitrogen replacement protection, then Start stirring and cool down to ≤-20°C, then replace nitrogen with hydrogen, keep the pressure in the kettle at 0.2MPa, and react at constant temperature and pressure for 8 hours. After the reaction is monitored by TLC, nitrogen is used for three times, and then the material is withdrawn and recovered by filtration The catalyst and filter cake were washed with methanol, the combined filtrates were concentrated under reduced pressure to recover methanol, and then vacuum-dried to ensure that the methanol content was less than 1% to obtain compound I crude product, white solid (1000 g, crude product yield 99%).
[0105] 2. Preparation of the target product androstenone
[0106] Compound I (500g, 1.724mol) was diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com