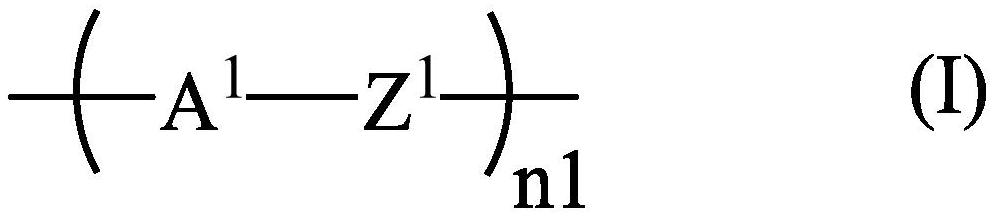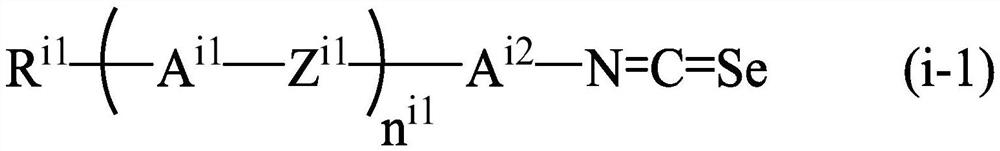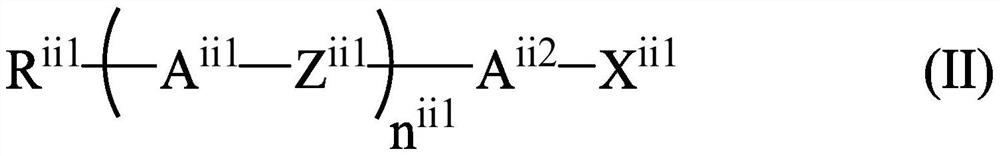Liquid crystal compound for high-frequency device
A technology of liquid crystal composition and compound, which is applied in the direction of liquid crystal materials, chemical instruments and methods, etc., can solve the problems of difficulty and insufficiency of liquid crystal composition, and achieve the effect of excellent electromagnetic wave control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0300] [Chemical 51]
[0301]
[0302] Under nitrogen atmosphere, for 4-iodoaniline (18.3g, 84mmol), triethylamine (10.2g, 100mmol), 1,8-diazabicyclo[5.4.0]-7-undecene ( 12.7g, 84mmol), tetrakis (triphenylphosphine) palladium (0.6g, 0.5mmol), copper (I) iodide (0.1g, 0.5mmol), THF (100mL) mixture, add 1-butane dropwise at room temperature A THF (40 mL) solution of 4-ethynylbenzene (14.6 g, 92 mmol) was stirred under reflux for 3 hours. The reaction solution was poured into water, the aqueous layer was extracted twice with toluene, the organic layers were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. After the solvent was distilled off under reduced pressure, 4-((4-butylphenyl)ethynyl)aniline (19.8 g, yield 95%) was obtained by recrystallization from hexane.
[0303] [Chemical 52]
[0304]
[0305] Under nitrogen atmosphere, a mixture of 4-((4-butylphenyl)ethynyl)aniline (18.8 g, 76 mmol), formic acid (10.4 g, 227 mmol), toluene (200 m...
Embodiment 2
[0310] [Chemical 54]
[0311]
[0312] Under nitrogen atmosphere, for 2-chloro-4-iodoaniline (21.3g, 84mmol), triethylamine (10.2g, 100mmol), 1,8-diazabicyclo[5.4.0]-7-deca A mixture of carbene (12.7g, 84mmol), tetrakis(triphenylphosphine) palladium (0.6g, 0.5mmol), copper (I) iodide (0.1g, 0.5mmol), THF (100mL), dropwise at room temperature A THF (40 mL) solution of 1-butyl-4-ethynylbenzene (14.6 g, 92 mmol) was added, and the mixture was stirred for 3 hours while heating to reflux. The reaction solution was poured into water, the aqueous layer was extracted twice with toluene, the organic layers were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. After the solvent was distilled off under reduced pressure, 4-((4-butylphenyl)ethynyl)-2-chloroaniline (22.6 g, yield 95%) was obtained by recrystallization from hexane.
[0313] [Chemical 55]
[0314]
[0315] A mixture of 4-((4-butylphenyl)ethynyl)-2-chloroaniline (22.6 g, 80 mmol), formic...
Embodiment 3
[0320] [Chemical 57]
[0321]
[0322]Under nitrogen atmosphere, for 4-iodoaniline (18.3g, 84mmol), triethylamine (10.2g, 100mmol), 1,8-diazabicyclo[5.4.0]-7-undecene ( 12.7g, 84mmol), tetrakis (triphenylphosphine) palladium (0.6g, 0.5mmol), copper (I) iodide (0.1g, 0.5mmol), THF (100mL) mixture, add 4-acetylene dropwise at room temperature A THF (40 mL) solution of 2-fluoro-1-propoxybenzene (16.4 g, 92 mmol) was stirred under reflux for 3 hours. The reaction solution was poured into water, the aqueous layer was extracted twice with toluene, the organic layers were combined, washed with saturated brine, and dried over anhydrous sodium sulfate. After the solvent was distilled off under reduced pressure, 4-((3-fluoro-4-propoxyphenyl)ethynyl)aniline (19.4 g, yield 86%) was obtained by recrystallization from hexane.
[0323] [Chemical 58]
[0324]
[0325] Under a nitrogen atmosphere, a mixture of 4-((3-fluoro-4-propoxyphenyl)ethynyl)aniline (19.4 g, 72 mmol), formic acid...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap