A kind of sustained-release preparation containing edoxaban and preparation method thereof
A technology for edoxaban and sustained-release preparations, applied in the field of edoxaban-containing sustained-release preparations and its preparation, to achieve the effects of enhanced blocking effect, increased viscosity, and optimized release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-11
[0032]
[0033]
[0034]
[0035]
[0036] Embodiment 1-11 preparation technology:
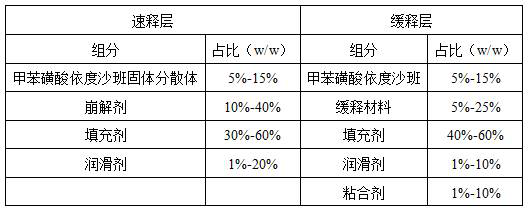
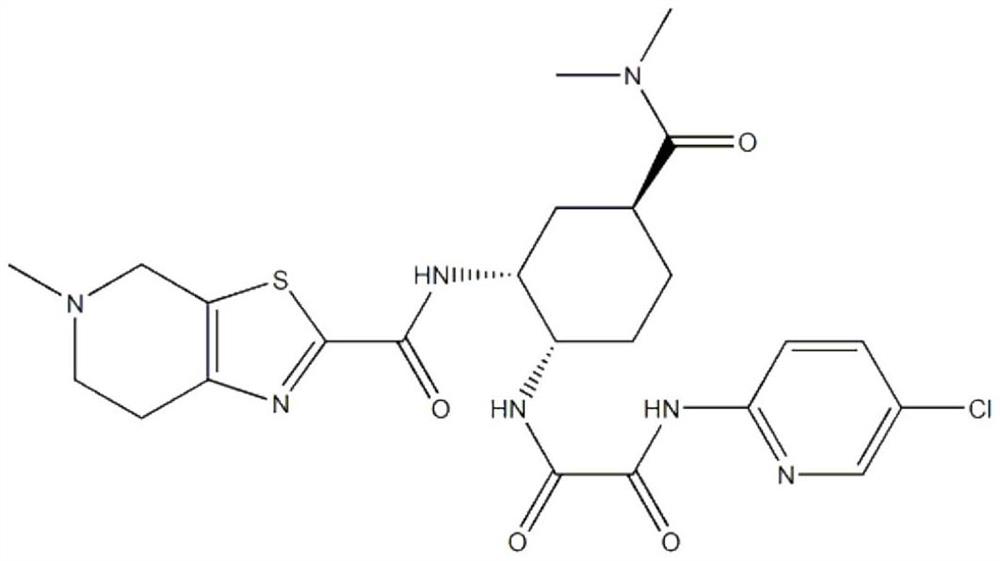
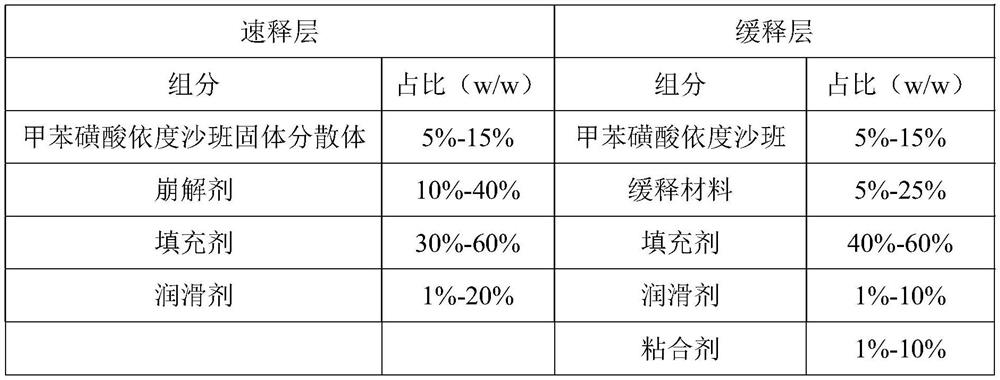
[0037] (a) Preparation of edoxaban solid dispersion: the weight component ratio of edoxaban tosylate, poloxamer and sodium lauryl sulfate is 1:1.5:0.5. Take edoxaban toluenesulfonate, add 10 times the amount of ethanol with a mass fraction of 80%, and ultrasonically assist in dissolving to obtain a homogeneous solution; take another poloxamer in an evaporating dish, heat it in a water bath, and pour it into toluene after it is completely melted Add edoxaban sulfonic acid solution, add sodium lauryl sulfate, stir evenly, evaporate the solvent, condense and stir the resulting mixture to solidify, dry, pulverize, and sieve;
[0038] (b) Preparation of the immediate-release layer: pass the edoxaban toluenesulfonate solid dispersion, the disintegrating agent and the filler in the prescribed amount through an 80-mesh sieve and mix uniformly, add magnesium stearate and mix uniformly to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com