A kind of stable polypeptide protein targeting inhibitor and use thereof
A peptide and protein target technology, applied in the field of bioengineering, can solve problems such as poor breast cancer effect, achieve significant technological progress, broaden the scope of application, and inhibit growth.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
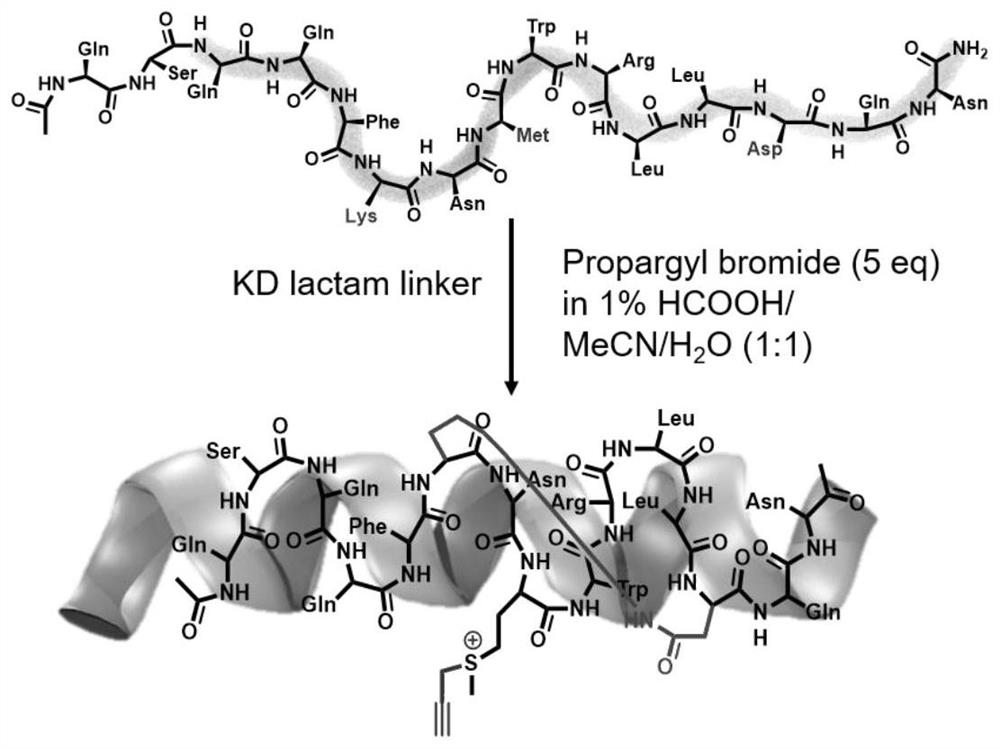
[0034]In order to better carry out the following research, the design of control peptides is necessary. As shown in Table 1, we designed a series of control peptides. The present invention selects SAH-p53-8 sequence (Ac-Q-S-Q-Q-T-F-*-N-X-W-R-L-L-#-Q-N-NH2) as the original sequence, and replaces it with methionine at its X position. And combined with the research strategy of Professor Wang Lei's research group (L. Wang, Chem. Commun., 2016, 52, 5140), they replaced the leucine at position 22 in the SAH-p53-8 sequence with an aromatic group containing leucine. The sulfonyl fluoride compound increased the inhibitory effect of SAH-p53-8 by 10 times. In the present invention, lysine and aspartic acid are used to close the side chain at the i position and the i+7 position, so that the polypeptide can be further stabilized. The synthesized polypeptide (1 equiv.) was reacted with bromopropyne reagent (5 equiv.) under acidic conditions for 12 hours to construct the polypeptides in Ta...
Embodiment 2
[0037] The preparation of embodiment 2 polypeptide and separation and purification steps:
[0038] The solid-phase synthesis of polypeptides according to the amino acid sequence, the core steps of preparing the above-mentioned stable polypeptides are as follows (take Peptide-2 as an example):
[0039]
[0040] The specific operation steps are:
[0041] (1) Polypeptide solid-phase synthesis: Weigh 100 mg of Rink amide MBHA resin into a 10 ml peptide tube, add dichloromethane (DCM), and swell with nitrogen for 30 min. A 50% (v / v) solution of morpholine in N,N-dimethylformamide (DMF) was added, and nitrogen was bubbled for 30 min to remove the Fmoc protecting group. After alternately washing the resin 6 times with DMF and DCM, the prepared (1) Fmoc-Asn-OH (5eq, 0.4M, DMF) solution, 6-chlorobenzotriazole-1,1,3,3-tetramethyl Urea hexafluorophosphate (HCTU) (5eq, 0.38M, DMF) solution, N,N-diisopropylethylamine (DIPEA) (10eq) were mixed, and then added to the resin with nitrogen...
Embodiment 3
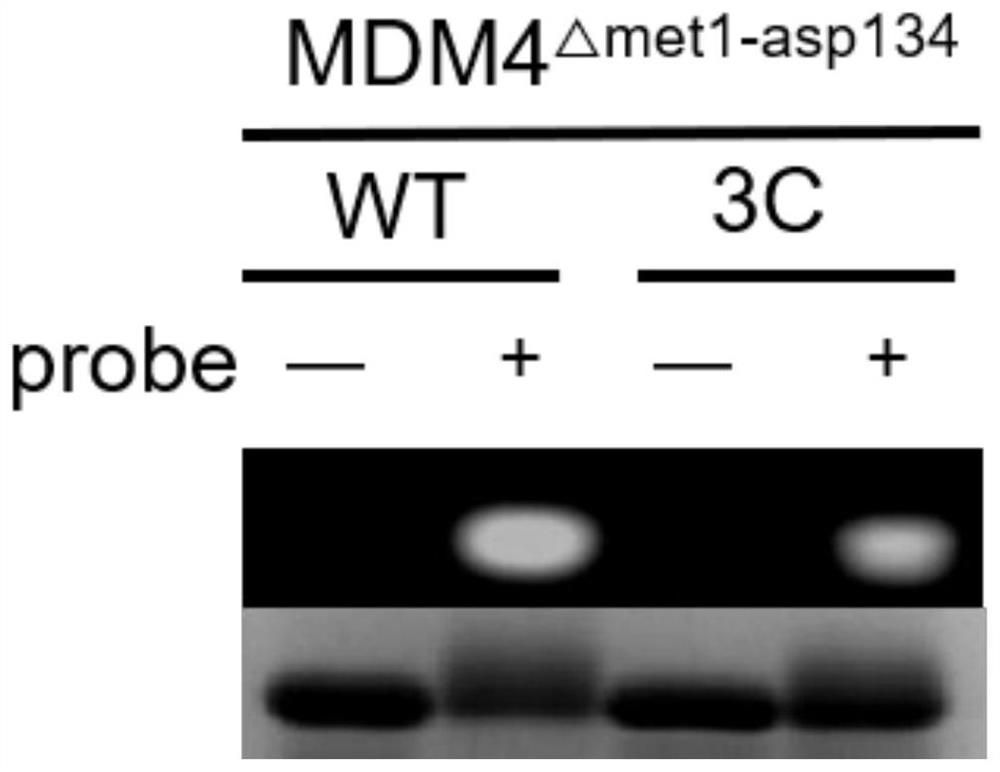
[0047] Example 3 Experiment of sulfonium salt-alkyne and lysine reaction
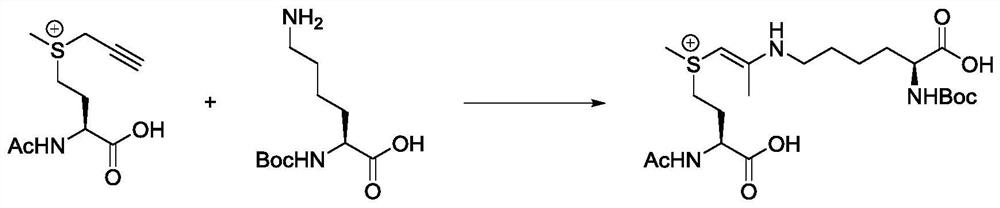
[0048] The present invention designs a model reaction, selects propargyl dimethyl sulfonium salt and Boc-Lys-OH as substrates to react in water for 12 hours, and then purifies by HPLC to obtain the target product. The present inventors carried out careful nuclear magnetic resonance (NMR) experiments, including 1H NMR, 13C{1H}NMR, heteronuclear single quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC) and total correlation spectroscopy (TOCSY), It can be confirmed that propargyl sulfonium salts can react with amino groups on lysine (e.g. Figure 4 ). Figure 4 A: 1H NMR: 1 H NMR(400MHz DMSO-d6)δ7.85-7.32(m,1H),6.29(d,J=7.5Hz,1H),4.39(s,1H),3.90-3.57(m,1H),2.83(s ,7H),2.05(s,3H),1.34(m,17H); Figure 4 B: 13 C{ 1 H}NMR: 13 C{ 1 H}NMR(400MHz DMSO-d6)δ174.7,158.6,158.5,158.2,155.3,118.8,115.8,77.6,69.1,54.6,52.0,44.9,42.9,31.9,31.5,28.3,27.1,23.1,18.1,8.8,7.7 ; Figure 4 C: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com