Reduction and pH super-sensitive cross-linked polymer prodrug and preparation method and application of polymer prodrug
A technology of cross-linking polymers and polymers, which is used in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problem of capillary hyperplasia, which is difficult to supply sufficient oxygen and nutrients. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] A reduction and pH ultra-sensitive cross-linked polymer prodrug, its structure is shown in formula III:
[0075]
Embodiment 2
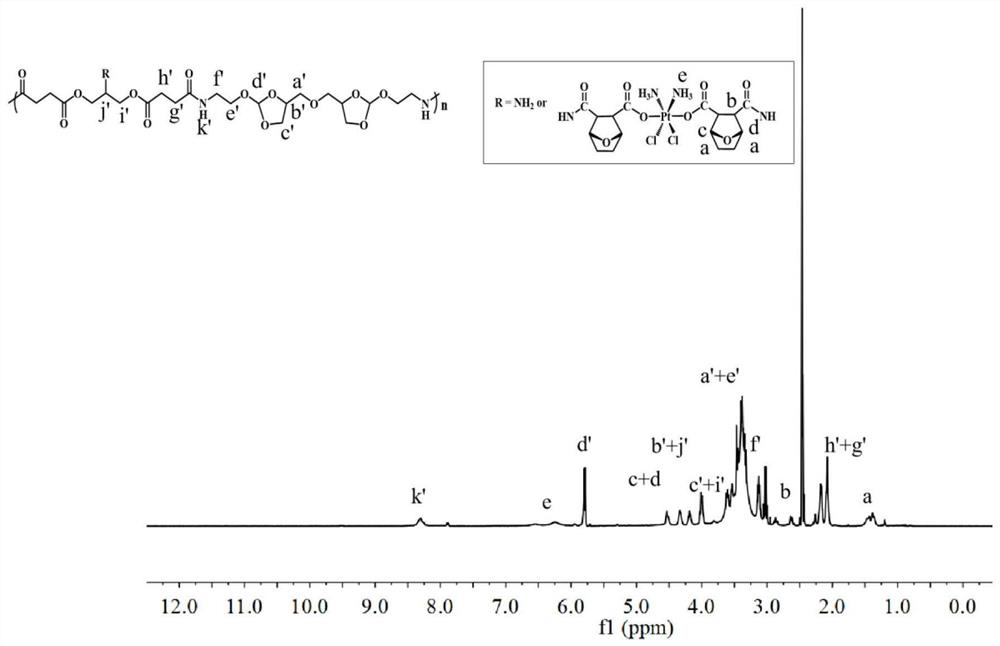
[0077] Preparation of reduction and pH ultra-sensitive cross-linked polymer prodrugs, the synthetic route is as follows:
[0078]
[0079] The preparation steps of reduction and pH ultrasensitive cross-linked polymeric prodrugs are as follows:
[0080] Formula I synthetic steps are as follows:
[0081]
[0082] The compound represented by formula I in this example and the preparation steps of the compound represented by formula I are the same as the synthesis steps in the patent with publication number CN105949467A.
[0083] Formula II synthetic route is as follows:
[0084]
[0085] The specific synthesis steps of formula II are as follows:
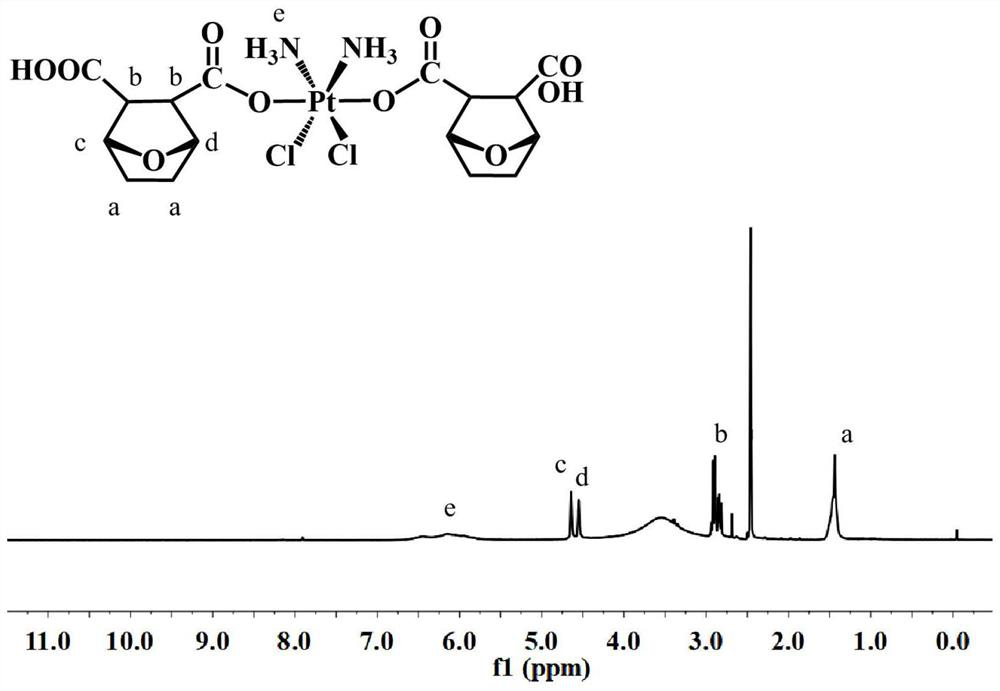
[0086] H 2 o 2 Dihydroxycisplatin (500mg, 1.493mmol), norcantharidin (1004mg, 5.972mmol) and triethylamine (catalytic amount) obtained by oxidation of the aqueous solution were dissolved in DMF, stirred at 65°C for 24 hours, and the solvent was distilled off under reduced pressure . Finally, the cisplatin-norcantharidin co...
Embodiment 3
[0091] Preparation of reduction and pH ultra-sensitive cross-linked polymer prodrug micelles and detection of particle size stability and critical micelle concentration:
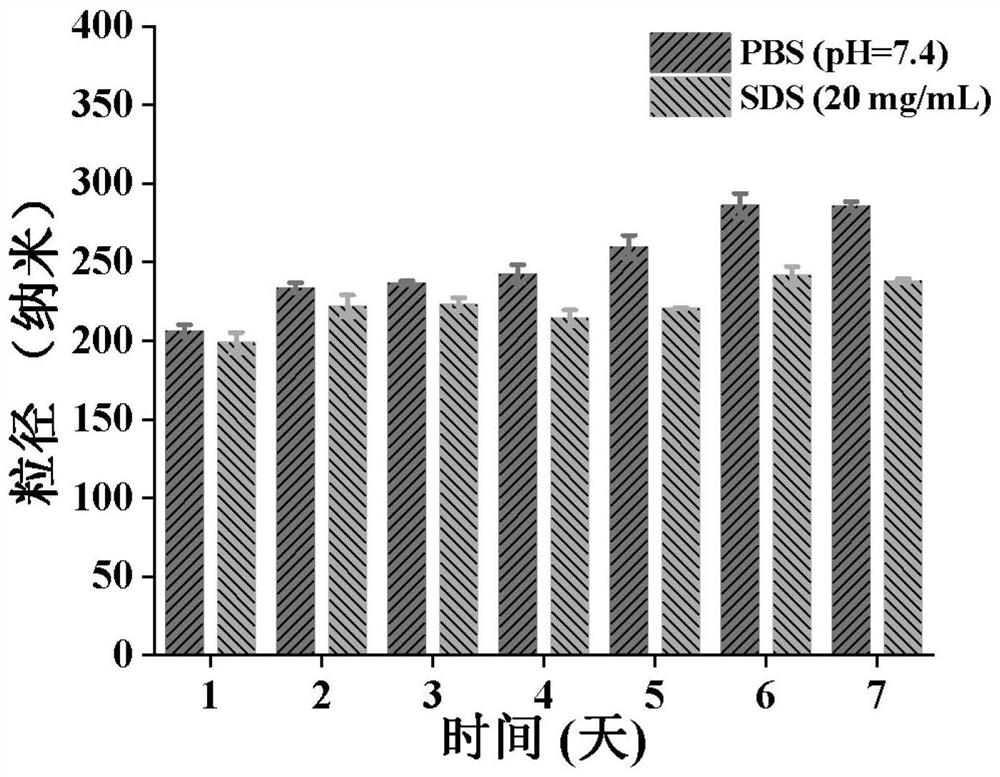
[0092] After 50 mg of the cross-linked polymer prodrug was added to 0.5 mL of DMSO to fully dissolve, it was added dropwise into phosphate buffer (20 mL, pH 7.4, 0.01 M) and stirred for 6 h. Then, the mixture was dialyzed against deionized water (MWCO 3500Da) for 24 h, and cross-linked micelles were obtained after lyophilization.
[0093] The cross-linked micelles were dispersed in 0.01M pH 7.4 phosphate buffer and 20mg / mL SDS solution, and the particle size was measured by DLS (Malvern, Zeta-sizer Nano-ZS90) at room temperature, once a day , measured for 7 days, the particle size stability is as follows image 3 shown.
[0094] Nile red was used as a probe, and the critical micelle concentration was determined by fluorescence spectrophotometer. Under light-shielded conditions, the cross-linked micelles w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com