Cryopreservation liquid for long-term preservation of human umbilical cord mesenchymal stem cells
A technology for long-term preservation of stem cells, which is applied in the field of cryopreservation for long-term preservation of human umbilical cord mesenchymal stem cells, which can solve the problems of unclear cell growth factors, pathogen cross-infection, cytotoxicity, etc., and improve safety and biological quality. Capacitive, reduced damage, less toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
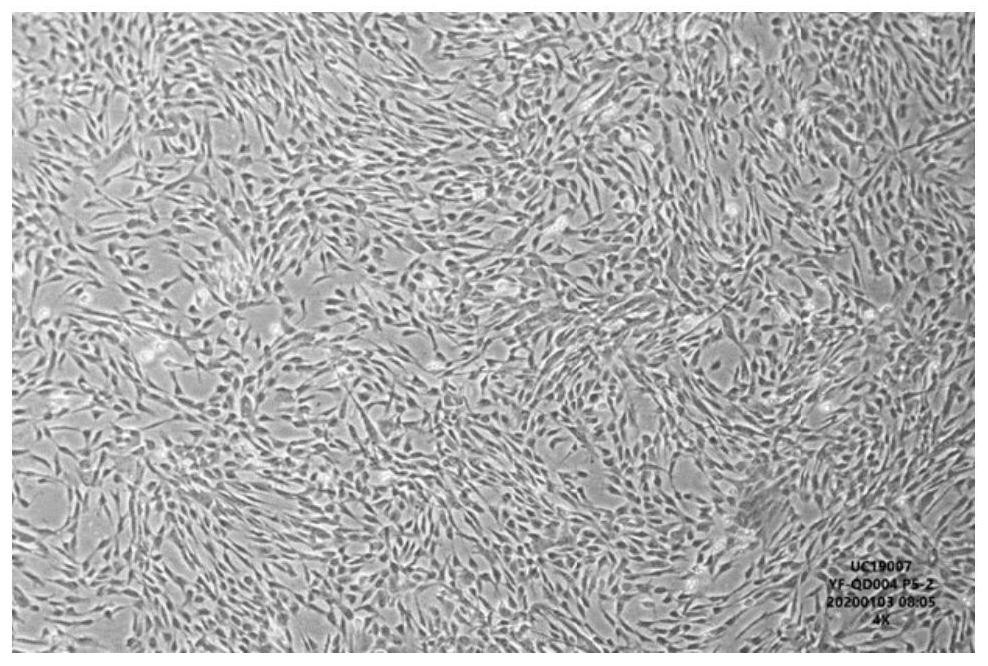
Image
Examples
preparation example Construction
[0071] The second aspect of the present invention provides the preparation method of the cryopreservation solution for long-term preservation of human umbilical cord mesenchymal stem cells, comprising the following steps: adding pre-cooled compound electrolyte injection, chlorinated Sodium injection, trehalose solution, dextran, and vitamin C injection, mix well; then add pre-cooled human serum albumin, mix well, and get ready.
[0072] In a preferred embodiment, after the cryopreservation solution is prepared, a pre-cooling step is also included.
[0073] In a preferred embodiment, the pre-cooling is at 2-8°C for at least 30 minutes.
[0074] In a most preferred embodiment, the preparation method of the human umbilical cord mesenchymal stem cell injection cryopreservation solution comprises the following steps: slowly adding pre-cooled compound electrolyte injection, chlorinated Sodium injection, trehalose solution, dextran, vitamin C injection and heparan sulfate, mix well;...
Embodiment 1
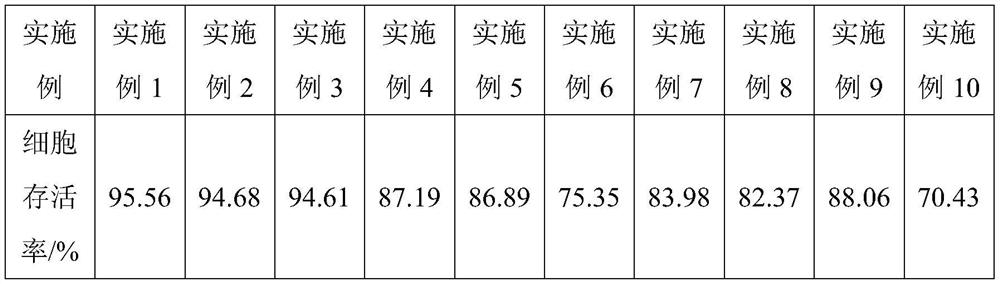
[0080] Example 1 provides a cryopreservation solution for long-term preservation of human umbilical cord mesenchymal stem cells. The raw materials for the preparation of the cryopreservation solution, by volume percentage, at least include the following components: dimethyl sulfoxide 3%, 20% human serum albumin 20%, 0.9% sodium chloride injection 3%, 1mol / L trehalose solution 6%, dextran-40 glucose injection 24%, vitamin C injection 2%, human α1-acid Glycoprotein 25 μg / mL, heparan sulfate 25 μg / mL, compound electrolyte injection to make up the balance.
[0081] The preparation method of the human umbilical cord mesenchymal stem cell injection cryopreservation solution comprises the following steps: slowly adding pre-cooled compound electrolyte injection, 0.9% sodium chloride injection, 1mol / L seaweed injection to dimethyl sulfoxide Sugar solution, dextran-40 glucose injection 24%, vitamin C injection and heparan sulfate, mix well; then slowly add pre-cooled human serum albumin...
Embodiment 2
[0083] Example 2 provides a cryopreservation solution for long-term preservation of human umbilical cord mesenchymal stem cells. The raw materials for the preparation of the cryopreservation solution, by volume percentage, at least include the following components: dimethyl sulfoxide 3%, 20% human serum albumin 10%, 0.9% sodium chloride injection 1%, 1mol / L trehalose solution 1%, dextran-40 glucose injection 5%, vitamin C injection 1%, human α1-acid Glycoprotein 20 μg / mL, heparan sulfate 400 μg / mL, compound electrolyte injection to make up the balance.
[0084] The preparation method of the human umbilical cord mesenchymal stem cell injection cryopreservation solution comprises the following steps: slowly adding pre-cooled compound electrolyte injection, 0.9% sodium chloride injection, 1mol / L seaweed injection to dimethyl sulfoxide Sugar solution, dextran-40 glucose injection 24%, vitamin C injection and heparan sulfate, mix well; then slowly add pre-cooled human serum albumin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com