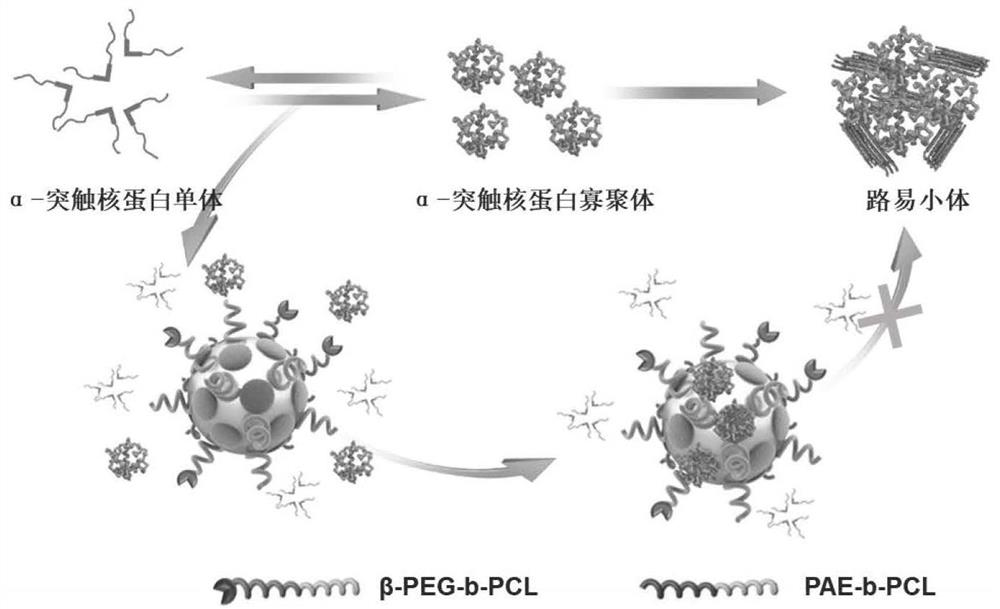
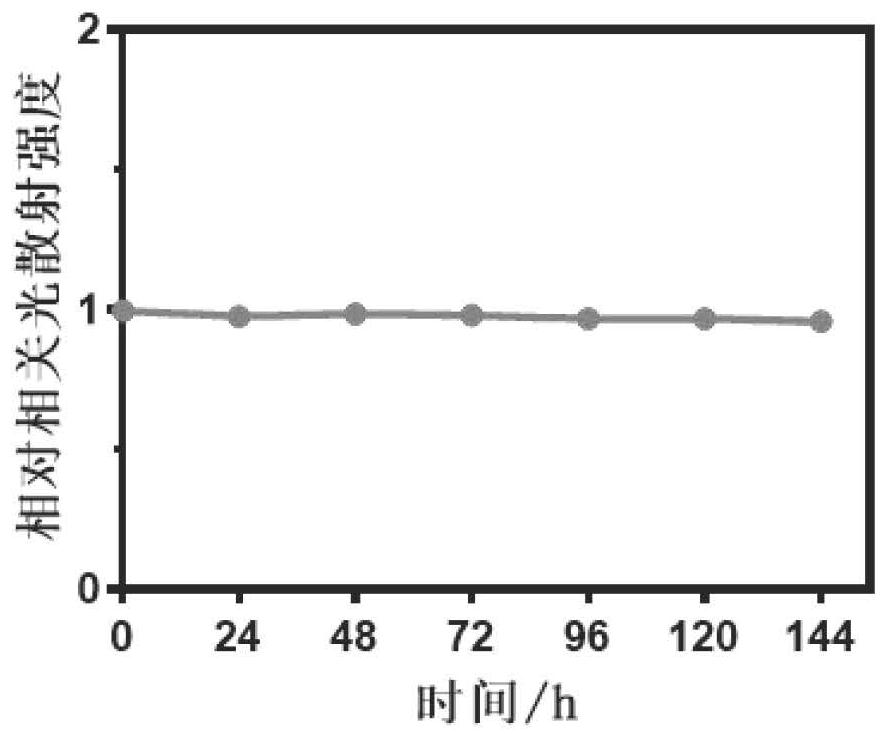
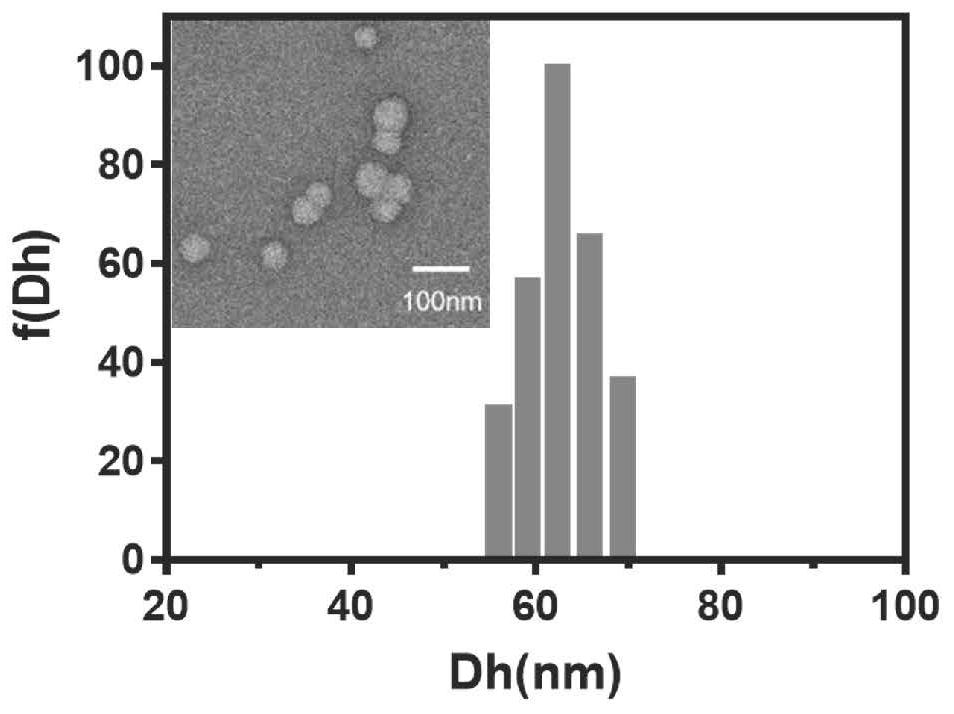
Preparation method and application of polypeptide functionalized composite micelle for inhibiting aggregation of alpha-synuclein
A technology of synuclein and composite micelles, which is applied in the field of preparation of composite micelles, and can solve the problems of poor remote adsorption effect and non-specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of a polypeptide-functionalized composite micelle that inhibits α-synuclein aggregation.
[0031] 1) Synthesis of βsyn36-PEG-b-PCL
[0032] Select βsyn36 (polypeptide sequence: GVLYVGSKTR) as the specific recognition peptide, dissolve 90mg of MAL-PEG-PCL and 20mg of polypeptide (C-GG-GVLYVGSKTR) in DMF treated by vacuum distillation, add 50ul of triethylamine, at room temperature The reaction was carried out under stirring for 24 hours. After the reaction was completed, the reaction solution was transferred to a dialysis bag for dialysis, and lyophilized to obtain βsyn36-PEG-PCL.
[0033] 2) Synthesis of PCL-b-PAE
[0034] Weigh 80 mg of hydroxyethyl acrylate (HMA) and 4 g of ε-CL, add them to an eggplant-shaped bottle, add 10 ml of toluene to dissolve, add 2 drops of stannous octoate as a catalyst, freeze in liquid nitrogen-fill with nitrogen-thaw for three times, and react in The reaction was carried out in an oil bath at 110°C for 12 hours, a...
Embodiment 2
[0038] Example 2: Preparation of a polypeptide-functionalized composite micelle that inhibits α-synuclein aggregation.
[0039] 1) Synthesis of βsyn36-PEG-b-PCL
[0040] Select βsyn36 (polypeptide sequence: GVLYVGSKTR) as the specific recognition peptide, dissolve 90mg of MAL-PEG-PCL and 20mg of polypeptide (C-GG-GVLYVGSKTR) in DMF treated by vacuum distillation, add 50ul of triethylamine, at room temperature The reaction was carried out under stirring for 24 hours. After the reaction was completed, the reaction solution was transferred to a dialysis bag for dialysis, and lyophilized to obtain βsyn36-PEG-PCL.
[0041] 2) Synthesis of PCL-b-PAE
[0042] Weigh 80 mg of hydroxyethyl acrylate (HMA) and 4 g of ε-CL, add them to an eggplant-shaped bottle, add 10 ml of toluene to dissolve, add 2 drops of stannous octoate as a catalyst, freeze in liquid nitrogen-fill with nitrogen-thaw for three times, and react in The reaction was carried out in an oil bath at 110°C for 12 hours, and...
Embodiment 3
[0046] Example 3: Preparation of a polypeptide-functionalized composite micelle that inhibits α-synuclein aggregation.
[0047] 1) Synthesis of βsyn36-PEG-b-PCL
[0048] Select βsyn36 (polypeptide sequence: GVLYVGSKTR) as the specific recognition peptide, dissolve 90mg of MAL-PEG-PCL and 20mg of polypeptide (C-GG-GVLYVGSKTR) in DMF treated by vacuum distillation, add 50ul of triethylamine, at room temperature The reaction was carried out under stirring for 24 hours. After the reaction was completed, the reaction solution was transferred to a dialysis bag for dialysis, and lyophilized to obtain βsyn36-PEG-PCL.
[0049] 2) Synthesis of PCL-b-PAE
[0050] Weigh 80 mg of hydroxyethyl acrylate (HMA) and 4 g of ε-CL, add them to an eggplant-shaped bottle, add 10 ml of toluene to dissolve, add 2 drops of stannous octoate as a catalyst, freeze in liquid nitrogen-fill with nitrogen-thaw for three times, and react in The reaction was carried out in an oil bath at 110°C for 12 hours, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com