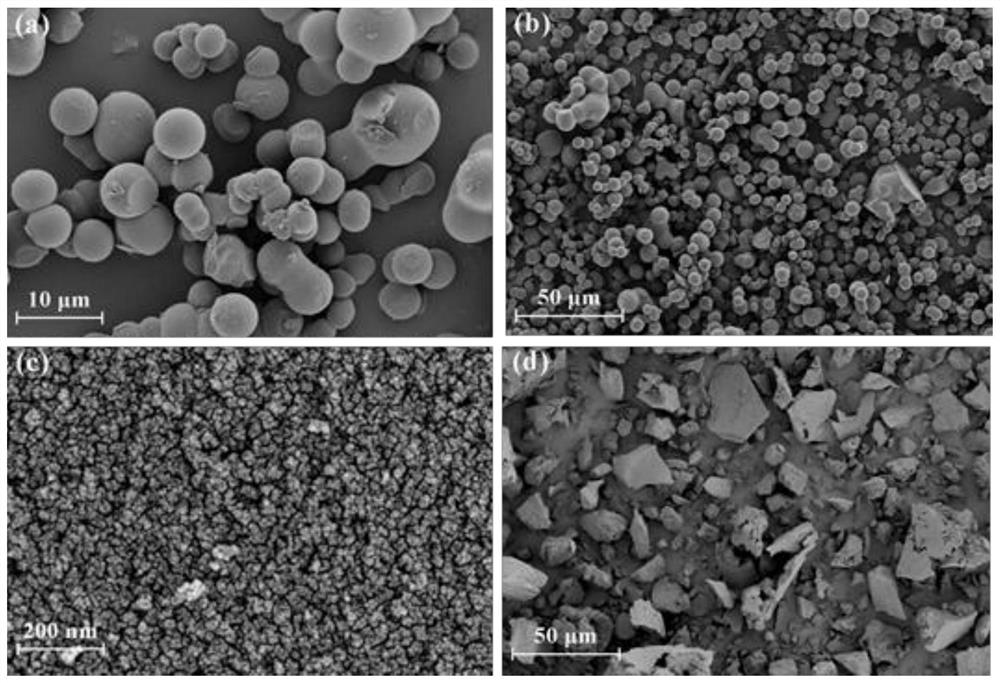
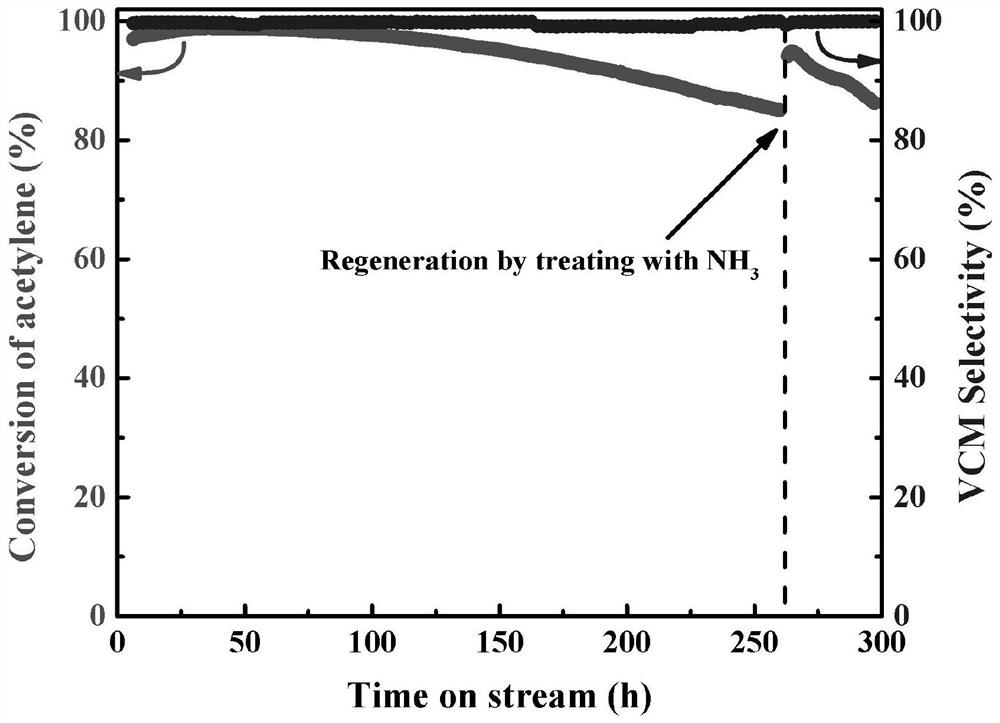
Nonmetal catalyst for acetylene hydrochlorination reaction and preparation method thereof
A non-metallic catalyst, acetylene hydrochlorination technology, applied in physical/chemical process catalysts, chemical instruments and methods, hydrogen halide addition preparation and other directions, can solve problems such as pollution, achieve low preparation cost, convenient operation and simple preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Operation steps for preparing catalyst:
[0051] Add 10g of D-(+)-glucose to 25mL of 1M hydrochloric acid, stir to form a transparent solution, then add m-phenylenediamine and ammonium persulfate in a molar ratio of 1:1 to the clear solution, and the solution is kept at 60°C Stir for 10 min. (The molar ratio of m-phenylenediamine to D-(+)-glucose is 0.3:1)
[0052] Then the solution was added into a stainless steel hydrothermal kettle for hydrothermal treatment, kept at 160° C. for 10 h, and then filtered to obtain a sample. After the sample was washed with water and ethanol for 10 h, it was suction filtered, and the sample obtained by suction filtration was placed in an oven and dried at 80° C. for 24 h. Control nitrogen and carbon. In inert gas N 2 Under atmosphere, the temperature was raised from room temperature to 800° C. at a rate of 5° C. / min, and kept for 2 hours to obtain the non-metallic catalyst. The obtained metal-free catalyst was named 0.3mPDA-C-800. ...
Embodiment 2
[0060] The amines used in this embodiment are o-phenylenediamine, m-phenylenediamine and p-phenylenediamine respectively. Other preparations and reaction conditions are the same as in Example 1. The conversion rate of vinyl chloride is shown in Table 1.
[0061] Table 1 Effect of different amine species on acetylene hydrochlorination
[0062] O-phenylenediamine m-phenylenediamine p-phenylenediamine Vinyl chloride conversion (%) 64.58 96.41 76.41
[0063] From the data in Table 1, it can be concluded that the conversion rate of vinyl chloride presents different conversion rates with the addition of different amine species. Among them, the addition of m-phenylenediamine has the most obvious promoting effect on the hydrochlorination of acetylene.
Embodiment 3
[0065] In this example, the molar ratios of phenylenediamine and ammonium persulfate are 1:0.5, 1:1, 1:1.5, and 1:2, respectively. Other preparations and reaction conditions are the same as in Example 1. The conversion rate of vinyl chloride is shown in Table 2.
[0066] The impact of table 2 m-phenylenediamine and ammonium persulfate molar ratio on acetylene hydrochlorination
[0067] The molar ratio of m-phenylenediamine and ammonium persulfate 1:0.5 1:1 1:1.5 1:2 Vinyl chloride conversion (%) 95.87 96.41 85.38 83.68
[0068] From the data in Table 2, it can be concluded that with the increase of ammonium persulfate addition, the conversion rate of vinyl chloride presents a trend of first increasing and then decreasing. Among them, when the ratio of m-phenylenediamine and ammonium persulfate is 1:1, the effect of promoting the hydrochlorination of acetylene is the most obvious.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com