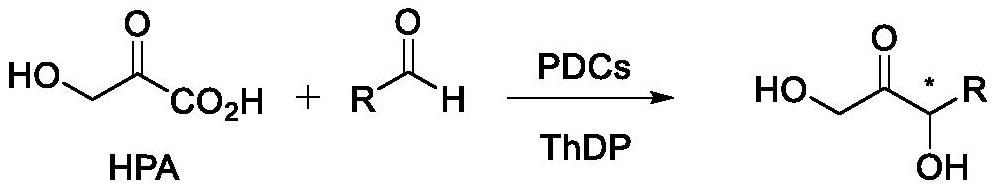
Synthesis method of chiral 1, 3-dihydroxy-1-aryl acetone compound
A technology of aryl acetone and hydroxypyruvate, applied in the field of biocatalysis, can solve the problems of low activity and narrow transketolase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Construction and culture of genetically engineered bacteria
[0023]The specific construction and cultivation method of the genetically engineered bacteria producing pyruvate decarboxylase is as follows: synthesizing the pyruvate decarboxylase nucleic acid sequence SEQ ID NO: 3 derived from Macrococcushajekii, constructing it into a pET series vector, in the host bacteria Escherichia coli Heterologous expression was performed. Thaw the strains stored at -80°C, streak the plates, and place them in a constant temperature incubator at 37°C for overnight culture. Select a single colony on the plate and inoculate it into 20 mL of LB medium containing the corresponding antibiotics, cultivate it for about 12 hours as the seed solution, and inoculate it into 700 mL of the LB medium containing the corresponding antibiotics according to the inoculum of 1%. Cultivated to OD 600 =0.6-0.8, IPTG with a final concentration of 0.1 mmol / L was added for induction at 25°C for...
Embodiment 2
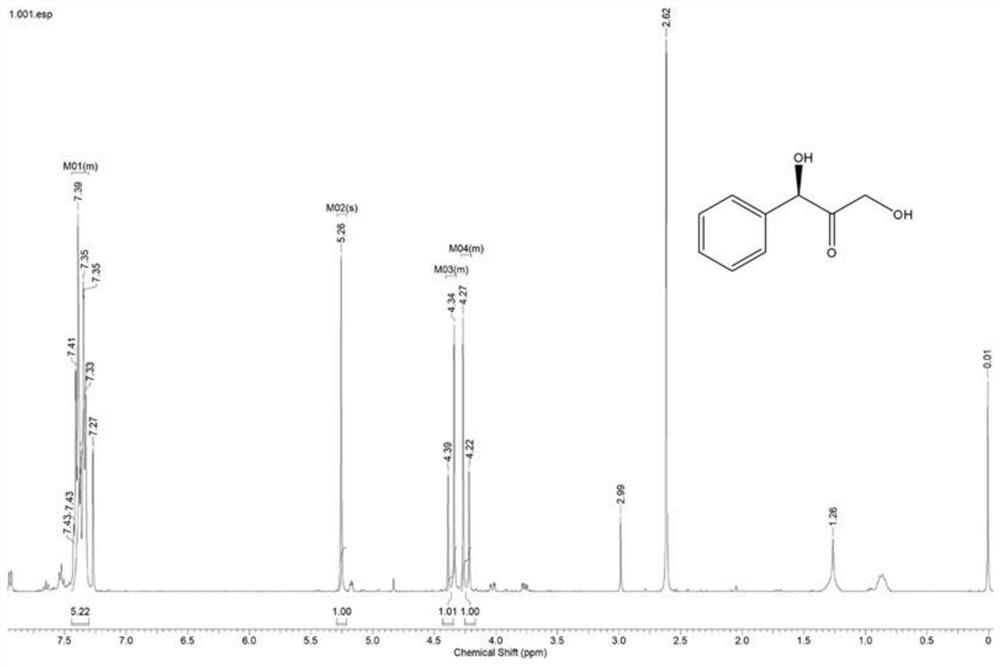
[0025] Example 2: Pyruvate decarboxylase catalyzes the preparation of 1,3-dihydroxy-1-phenylacetone from benzaldehyde and hydroxypyruvate.
[0026] (1) Add 0.26g hydroxypyruvate (25mM), 0.01g ThDP (0.1mM), 3.00g pyruvate decarboxylase genetically engineered bacteria, 20mM benzaldehyde (dissolved in 20% DMSO) into 80mL water, and adjust the pH of the reaction system to 8.0, react at 25°C for 24h. After the reaction, the protein was removed by centrifugation, the supernatant was extracted with ethyl acetate, and the extraction was complete by thin-layer chromatography. The organic solvent is removed by rotary evaporation to obtain the crude product, the substrate conversion rate and product yield are detected by gas phase, and the ratio of product and by-product isomers is detected by liquid phase HPLC. The detection result is: pyruvate decarboxylase catalyzes the R configuration product, the ee value is >99%, the conversion rate of benzaldehyde substrate is 61%, and the ratio ...
Embodiment 3
[0032] Example 3: Pyruvate decarboxylase and D-amino acid oxidase genetically engineered bacteria to prepare 1,3-dihydroxy-1-arylacetone.
[0033] 2.00g D-amino acid oxidase genetically engineered bacteria, 3.00g pyruvate decarboxylase genetically engineered bacteria, 1.05g D-Serine (100mM), 0.02g MgCl 2 ·6H 2 O (1mM), 0.01g ThDP (0.1mM), 60mg catalase (15KU) were added to 80mL water, the pH of the reaction system was adjusted to 7.0, incubated at 25°C, 200rpm for 1h, then added 20mM benzaldehyde (dissolved in 20% DMSO) and reacted for 24h . After the reaction, the protein was removed by centrifugation, the supernatant was extracted with ethyl acetate, and the extraction was complete by thin-layer chromatography. The organic solvent is removed by rotary evaporation to obtain the crude product, the substrate conversion rate and product yield are detected by gas phase, and the ratio of product and by-product isomers is detected by liquid phase HPLC. The detection results are:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com