Core-shell structure polymer magnetic nanosphere with high Cr(VI) adsorption capacity as well as preparation method and application thereof
A technology of polymer magnetism and core-shell structure, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve problems such as uneven distribution, increase specific surface area, excellent cyclic adsorption performance, and save energy The effect of separating costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
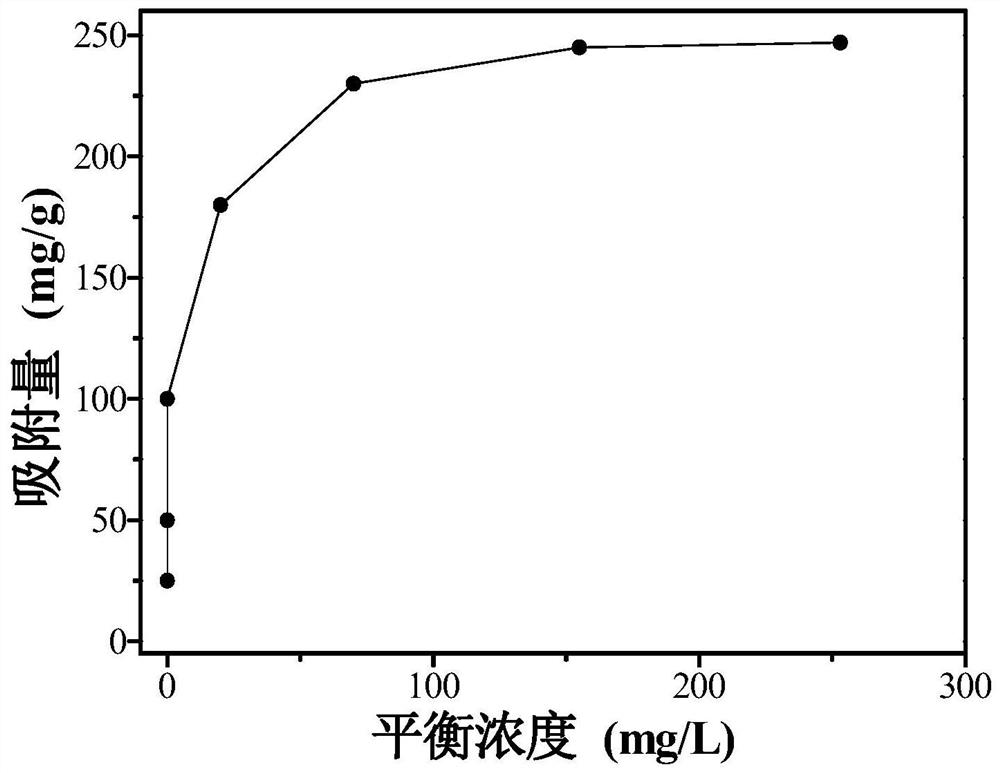
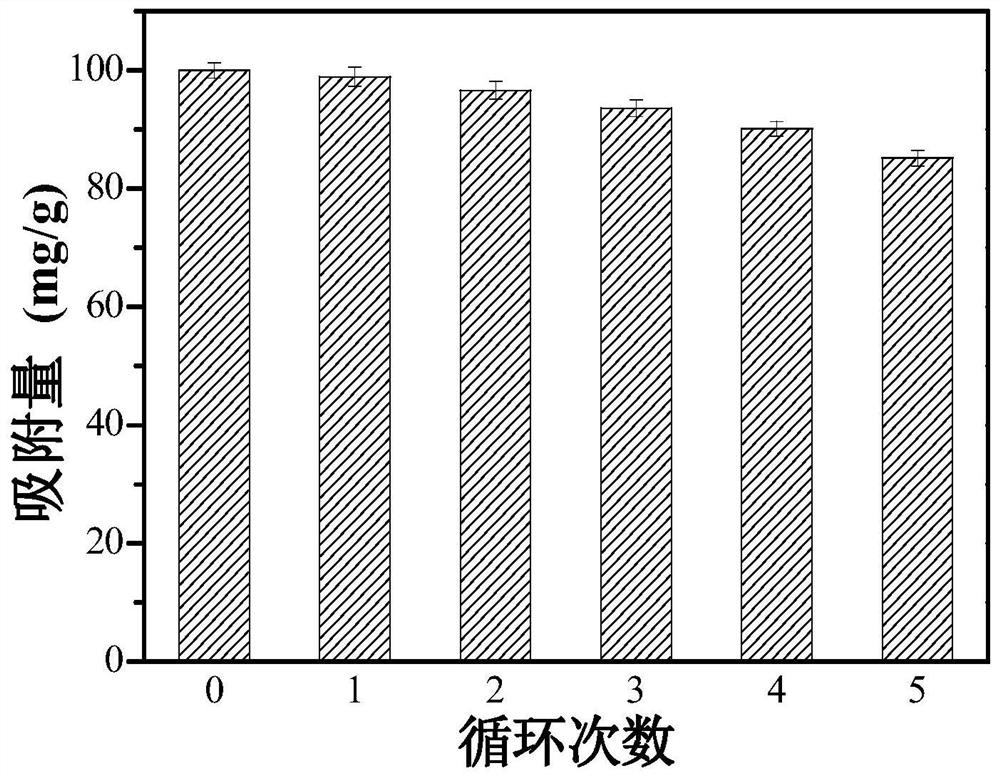
Examples
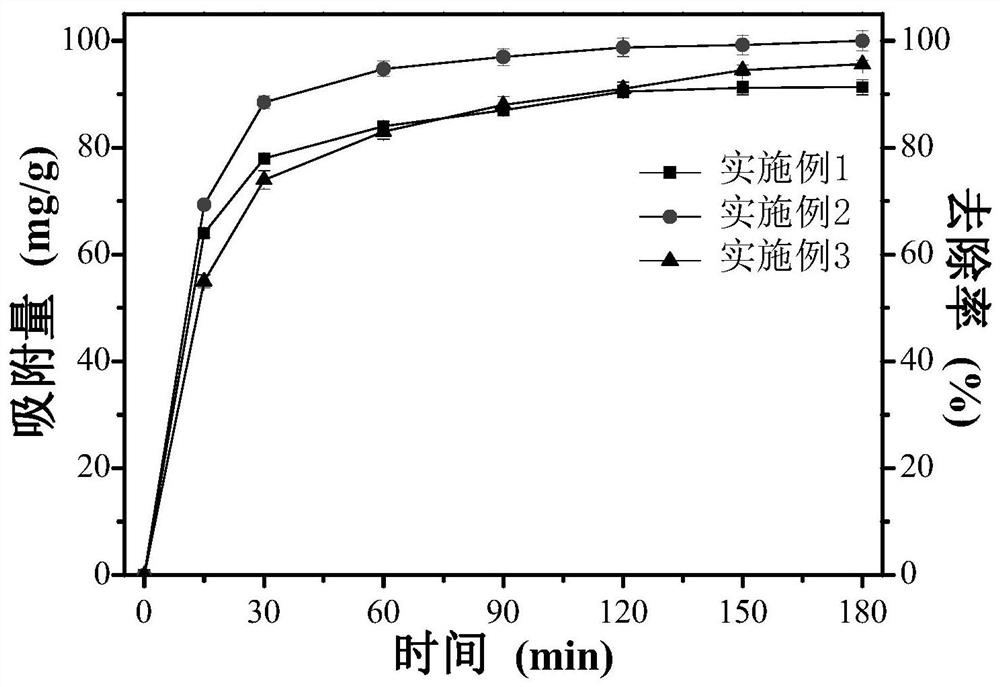
Embodiment 1
[0043] Dissolve 2.7g FeCl in 80mL ethylene glycol at room temperature 3 ·6H 2 O, stir magnetically until it is clear and transparent, then add 7.2g NaAc, and continue stirring until the solid is completely dissolved. The mixed solution was transferred into a 100mL polytetrafluoroethylene-lined reaction kettle, and kept in an oven at 200°C for 16h at a constant temperature. After cooling to room temperature, discard the supernatant, wash the black precipitate several times with ethanol until the liquid is clear, separate the product with a permanent magnet, and then dry the solid under vacuum at 60°C for 12 hours to obtain Fe 3 o 4 Powder.
[0044] 0.5g Fe 3 o 4 Added to 20mL deionized water and 40mL ethanol, sonicated for 10min to make Fe 3 o 4 Evenly dispersed in the solution. Then 0.4 g of resorcinol and 0.8 g of formaldehyde were sequentially added to the suspension, the pH of the suspension was adjusted to 9 with aqueous ammonia, and finally the suspension was stir...
Embodiment 2
[0048] Dissolve 2.7g FeCl in 80mL ethylene glycol at room temperature 3 ·6H 2 O, stir magnetically until it is clear and transparent, then add 7.2g NaAc, and continue stirring until the solid is completely dissolved. The mixture was transferred into a 100mL polytetrafluoroethylene-lined reaction kettle, and kept in an oven at 200°C for 16h at a constant temperature. After cooling to room temperature, discard the supernatant, wash the black precipitate several times with ethanol until the liquid is clear, separate the product with a permanent magnet, and then vacuum dry the solid at 60°C for 12 hours to obtain Fe 3 o 4 Powder.
[0049] 0.5g Fe 3 o 4 Add to 20mL deionized water and 40mL ethanol, ultrasonic 13min to make Fe 3 o 4 Evenly dispersed in the solution. Then 0.4 g of resorcinol and 0.8 g of formaldehyde were added to the suspension in sequence, the pH of the solution was adjusted to 10 with ammonia water, and finally the mixed suspension was stirred at 30° C. fo...
Embodiment 3
[0053] Dissolve 2.7g FeCl in 80mL ethylene glycol at room temperature 3 ·6H 2 O, stir magnetically until it is clear and transparent, then add 7.2g NaAc, and continue stirring until the solid is completely dissolved. The mixed solution was transferred into a 100mL polytetrafluoroethylene-lined reaction kettle, and kept in an oven at 200°C for 16h at a constant temperature. After cooling to room temperature, discard the supernatant, wash the black precipitate several times with ethanol until the liquid is clear, and separate the product with a permanent magnet; vacuum-dry the filter cake at 60°C for 12 hours to obtain Fe 3 o 4 Powder.
[0054] 0.5g Fe 3 o 4 Add to 20mL deionized water and 40mL ethanol, ultrasonic 15min to make Fe 3 o 4 Evenly dispersed in the solution. Then 0.4 g of resorcinol and 0.8 g of formaldehyde were sequentially added to the suspension, the pH thereof was adjusted to 11 with aqueous ammonia, and finally the mixed suspension was stirred at 30° C....
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com