A kind of piracetam tablet and preparation method thereof
A technology for piracetam tablets and prescriptions, applied in the field of piracetam tablets and its preparation, can solve the problems of low bioavailability, low dissolution rate, long disintegration time limit, etc., and achieve good fluidity and compressibility , Improve stability, suitable and reasonable effect of prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1 Preparation of Piracetam Tablets
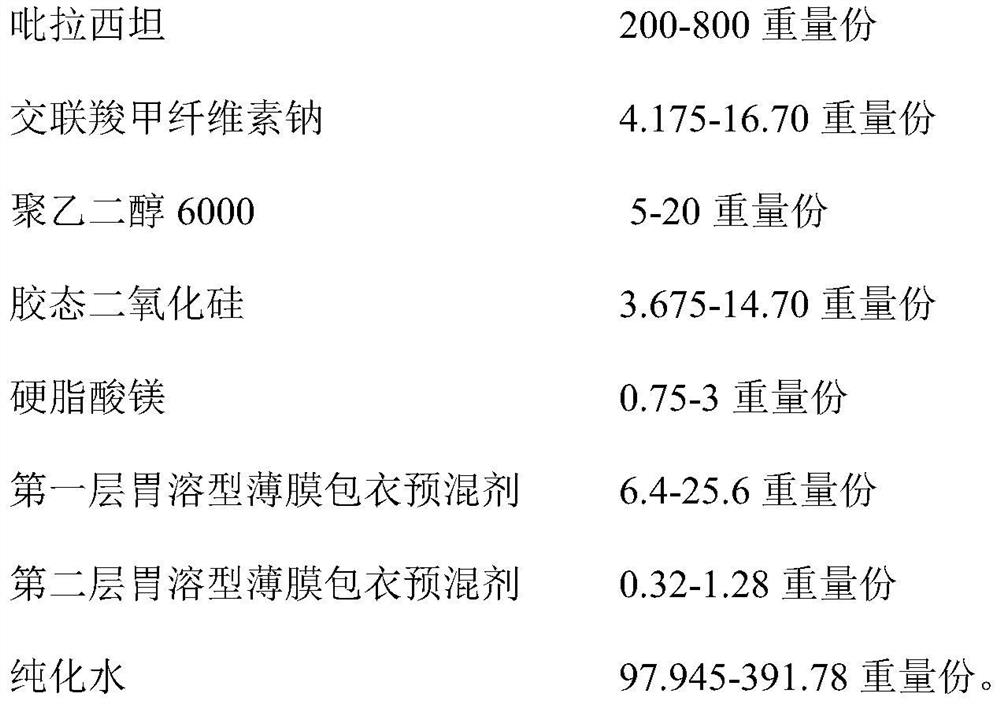
[0065] Prescription ingredients and dosage:
[0066] Element Preparation of 420,000 tablets parts by weight Piracetam 84.0kg 200 Croscarmellose sodium 1.75kg 4.175 polyethylene glycol 6000 2.1kg 5 Colloidal silica 3.86kg 3.675 Magnesium stearate 1.54kg 3 First-layer gastrosoluble film coating premix (white) 2.688kg 6.4 The second layer of gastrosoluble film coating premix (transparent color) 0.268 0.32 purified water 41.14kg 97.945
[0067] Preparation:
[0068] (1) Pretreatment: weigh the raw material of piracetam and add it to the universal pulverizer for pulverization. Sodium methylcellulose was passed through an 80-mesh sieve, for use;
[0069] (2) Premixing / granulation: Weigh the pretreated piracetam, croscarmellose sodium, internally added colloidal silicon dioxide (40% of the recipe amount), internally added polyethylene glycol 6000 (40%...
Embodiment 2
[0085] Example 2 Preparation of Piracetam Tablets
[0086] Prescription ingredients and dosage:
[0087] Element Preparation of 420,000 tablets parts by weight Piracetam 168kg 400 Croscarmellose sodium 3.5kg 8.33 polyethylene glycol 6000 4.2kg 10 Colloidal silica 3.09kg 7.36 Magnesium stearate 0.63kg 1.5 First-layer gastrosoluble film coating premix (white) 5.38 12.8 The second layer of gastrosoluble film coating premix (transparent color) 0.269 0.64 purified water 195kg 464.2
[0088] Preparation:
[0089] (1) Pretreatment: weigh the raw material of piracetam and add it to the universal pulverizer for pulverization. Sodium methylcellulose was passed through an 80-mesh sieve, for use;
[0090] (2) Premixing / granulation: Weigh the pretreated piracetam, croscarmellose sodium, add colloidal silicon dioxide (50% of the recipe), add polyethylene glycol 6000 (50% of the recipe quantity) was added into ...
Embodiment 3
[0106] Example 3 Preparation of Piracetam Tablets
[0107] Prescription ingredients and dosage:
[0108] Element Preparation of 420,000 tablets parts by weight Piracetam 252kg 600 Croscarmellose sodium 5.26kg 12.525 polyethylene glycol 6000 6.30kg 15 Colloidal silica 4.63kg 11.025 Magnesium stearate 3.78kg 9 First-layer gastrosoluble film coating premix (white) 8.06kg 19.2 The second layer of gastrosoluble film coating premix (transparent color) 0.40kg 0.96 purified water 123.4kg 293.835
[0109] Preparation:
[0110] (1) Pretreatment: weigh the raw material of piracetam and add it to the universal pulverizer for pulverization or sieving. The sieve aperture is 100 meshes, and D90 is required not to exceed 390 microns; polyethylene glycol 6000, colloidal silica, Cross-linked sodium carboxymethyl cellulose was passed through 80 mesh sieves for use;
[0111] (2) Premixing / granulation: Weigh the pretr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com