Anti-VIM carbapenemase hybridoma cell strain, monoclonal antibody and application
A hybridoma cell line and carbapenemase technology, which can be used in applications, anti-enzyme immunoglobulins, instruments, etc., can solve the problems of limited range of clinical treatment drugs, clinical infection control and treatment difficulties, etc. Clinical efficacy of infection control and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
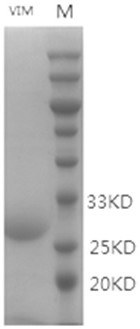
[0122] Embodiment 1: Preparation of anti-VIM type carbapenemase antibody
[0123] 1.1 Antigen preparation
[0124] Find and download the VIM-type carbapenemase gene sequence from NCBI, carry out the whole gene synthesis and connect it to the pet-28a vector, and transform the vector plasmid pet-28a-VIM containing the target gene into the expression host Rosseta (DE3) , the induction condition is that when the OD value of the bacterial solution is between 0.4 and 0.6, add IPTG to a final concentration of 1mM, incubate at 37 degrees for 3 hours, collect the bacterial cells by centrifugation, add about 1g of bacterial cells to 30mL PBS to resuspend the bacterial cells, and then ultrasonically break. The power is 400 W, ultrasonic 3 s, interval 5 s, after about 30 minutes, the bacterial solution is not viscous and clarified, centrifuge to remove the bacterial fragments, pass the supernatant through a 0.45 μm filter membrane, and load the sample until the filler is filled in advance...
Embodiment 2
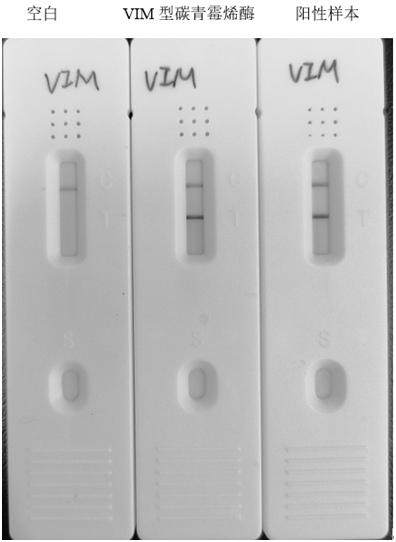
[0133] Example 2: Identification of anti-VIM-type carbapenemase antibodies
[0134] 2.1 Antibody subclass identification
[0135] According to the instructions of the SIGMA kit, the subclass identification of monoclonal antibody was carried out by the method of capture ELISA, as follows: After diluting the monoclonal antibody subclass identification reagent at 1:1000, add it to the enzyme-labeled well, 100 μL / well, and incubate at 37 ° C for 1 h; Wash three times with PBST, pat dry; add antibody after 1:1000 dilution, 100 μL / well, incubate at 37°C for 1 h; wash three times with PBST, pat dry; HRP enzyme-labeled goat anti-mouse IgG secondary antibody diluted 1:6000 Add sample, 100 μL / well, incubate at room temperature for 30 minutes; develop color for 10-20 minutes. The subtype of the monoclonal antibody belongs to the subtype whose OD450 reading value is significantly higher than that of the subtype reagent added to other wells. The antibody subtype of antibodies 1AF6 and 1E...
Embodiment 3
[0140] Example 3: Genetic verification of anti-VIM-type carbapenemase antibodies
[0141] Ig variable region genes were cloned by RT-PCR. Extract total RNA, synthesize single-stranded cDNA, extract total RNA from 1AF6 and 1EF3 hybridoma cell lines by Trizol method (kit purchased from Invitrogen), and reverse total RNA to cDNA with M-MLV reverse transcriptase (purchased from Invitrogen) library.
[0142] Heavy chain framework region upstream primer
[0143] P1: 5'SAGGTGMAGCTKCASSARTCWGG3'
[0144] Heavy chain variable region downstream primer
[0145] P2: 5'TGGGGSTGTYGTTTTGGCTGMRGAGACRGTGA3'
[0146] light chain leader peptide upstream primer
[0147] P3: 5'ATGGATTTTCAAGTGCAGATTTTCAG3'
[0148] Light chain variable region downstream primer
[0149] P4: 5'GGATACAGTTGGTGCAGCATCAGCCCGTTT3'
[0150] Prepare the PCR reaction system (50 μl) as follows:
[0151] cDNA: 2 μl; upstream primer (10 μM): 2 μl; downstream primer (10 μM): 2 μl; dNTP mixture: 2 μl; pfuDNA polymerase (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com