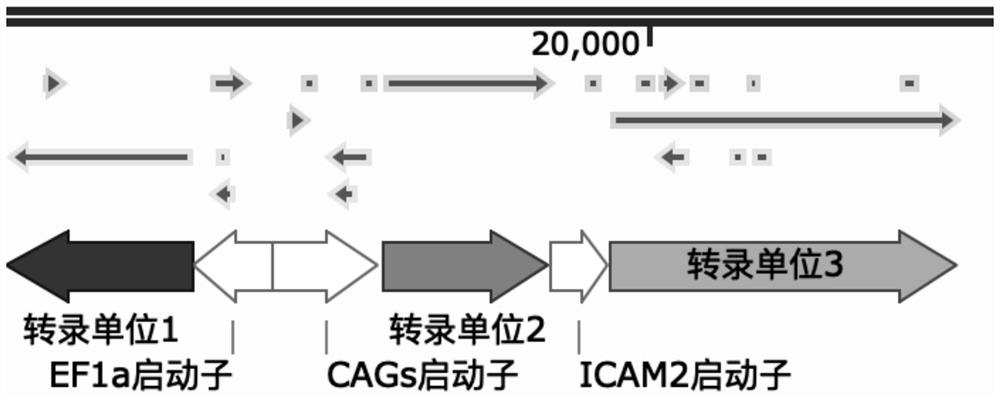
Active gene modified pig skin for covering and repairing human skin wounds and application of active gene modified pig skin
A technology for skin wounds and genetic modification, applied in skin transplantation, gene therapy, genetic engineering, etc., can solve problems such as loss of function, achieve the effect of promoting wound repair, solving the problem of inability to survive, and saving the lives of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
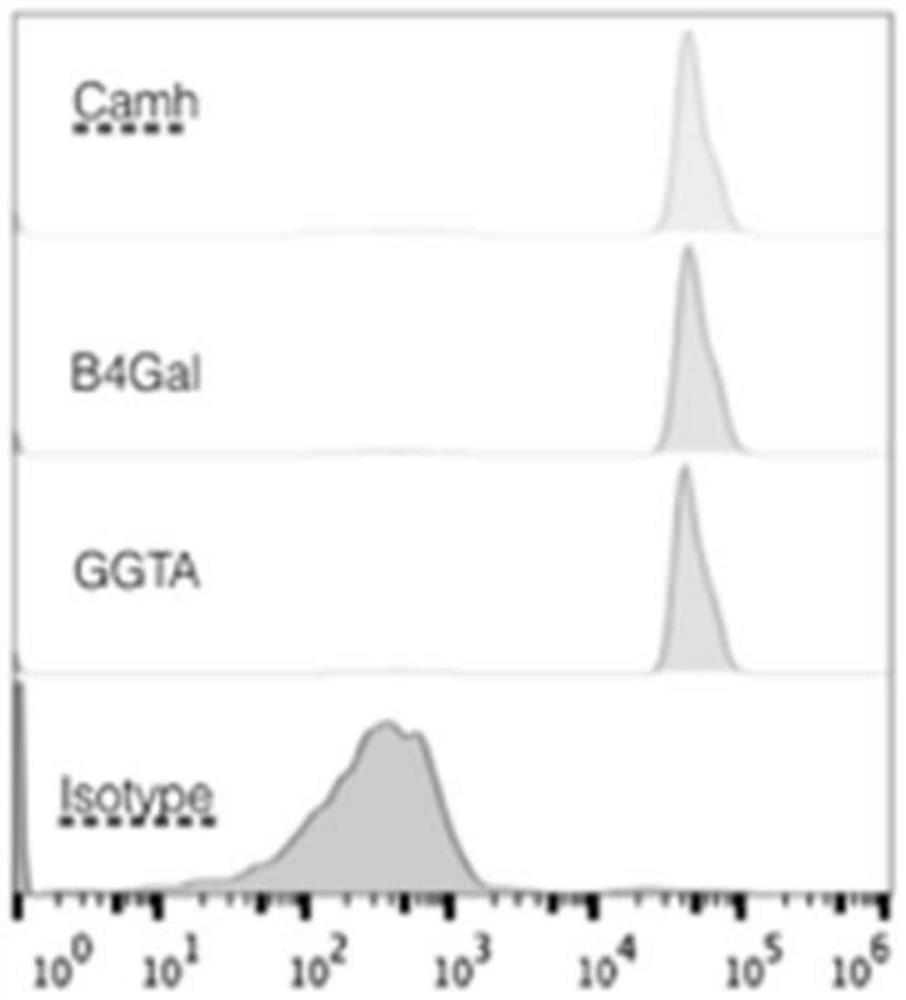
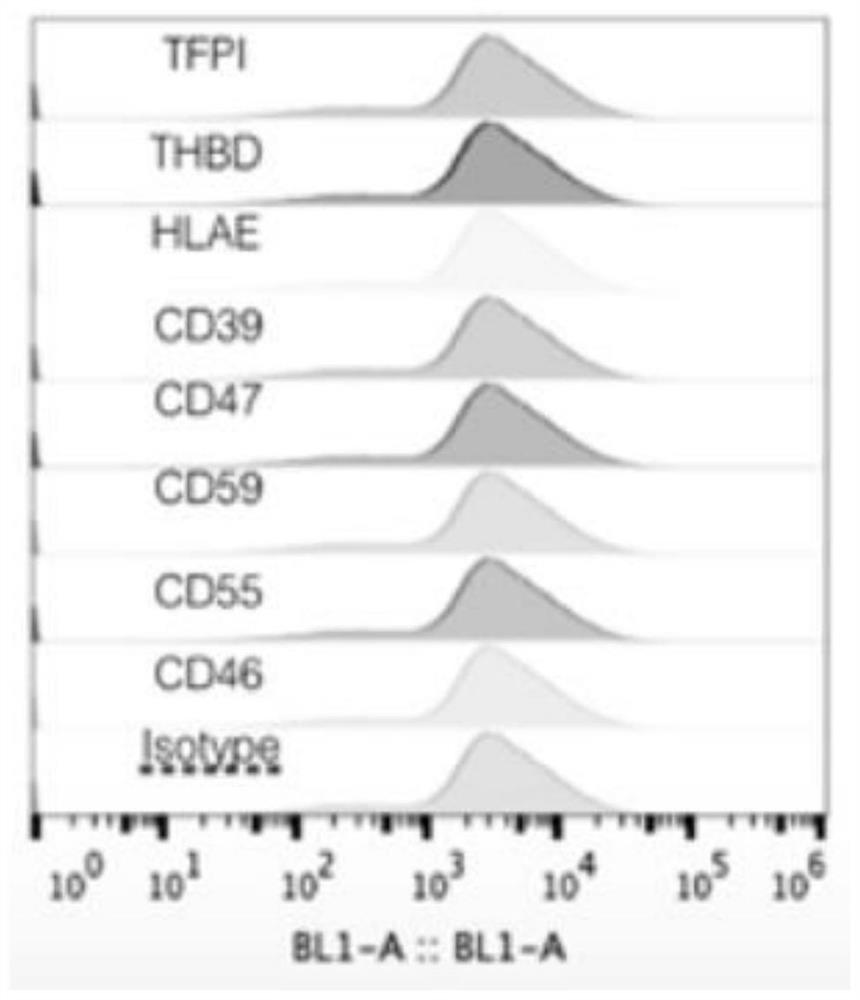
[0046] Antigen removal from isolated porcine skin and human protein expression and functional identification, Figure 2-5 The flow cytometry test indicated that the genetically modified pigs stably expressed human-derived CD55, CD59, CD46, CD47, CD39, THBD, TFPI and HLAE proteins, indicating that the above genes were successfully knocked in. The expression of GGTA, B4gal, and Camh proteins showed that the galactose antigen gene knockout was successful.
[0047] Figure 6-8 Antibody binding experiment of the mixed human serum shown: Pig ear fibroblasts were incubated with 30 healthy volunteers mixed serum at 37°C for 30 minutes with 75% (100% human mixed serum normal saline mass fraction diluted to 75% before the experiment), Wild-type pig cells carry galactose antigens that can be bound by pre-stored antibodies in human serum. Fluorescent antibody staining marks human IgM and IgG antibodies bound to pig cells, and the fluorescence intensity is measured by flow cytometry.
[...
Embodiment 2
[0051] Ex vivo porcine skin-Stone crab monkey skin xenograft
[0052] 1. Pig skin preparation: Inject phenobarbital sodium at a dose of 30 mg / Kg for anesthesia. After shaving, scrub repeatedly with soap and tap water, then routinely disinfect with povidone iodine and 70% alcohol by volume, spread towels, and take the back The skin is made of pigskin slices with a thickness of about 0.6mm using an electric skin remover, cut into 5cm×5cm skin slices, rinsed repeatedly with physiological saline containing antibiotics and placed in a 4-degree refrigerator for later use. In addition, fresh skin slices were infiltrated in an antifreeze protective agent containing 5% DMSO for 20 minutes, then taken out, packed into a sterile polyvinyl chloride pouch, sealed, and placed in a -80°C refrigerator or liquid nitrogen tank for later use. Before use, rewarm in a 37°C water bath and rinse with normal saline 3 times.
[0053] 2. Transplantation steps of genetically modified pig full-thickness...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com