A slow-release drug delivery system
A pharmaceutical and pharmaceutical technology, applied in the field of sustained-release drug delivery system composition and its preparation, can solve the problems of pain and irritation at the injection site, oil-gel preparations that have not been seen, preparation application limitations, etc., and achieve good biocompatibility sex, good drug safety and tolerability, and ease of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] The dissolving situation investigation of embodiment 1 different types of saturated phospholipids in castor oil and each organic solvent
[0103]Take 1g of castor oil and organic solvent respectively in EP tube, add 0.1g of saturated phospholipid (about 100mg / g, 10%w / w), ultrasonic at 50℃, observe the dissolution, the results are shown in Table 1-1.
[0104] Table 1-1 Dissolution investigation
[0105]
[0106] Wherein, EtOH stands for absolute ethanol; BB stands for benzyl benzoate; BA stands for benzyl alcohol; NMP stands for N-methylpyrrolidone; DMSO stands for dimethyl sulfoxide.
[0107] According to the solubility investigation, saturated phospholipids (HSPC, DPPC, DMPC, DSPC) have good solubility in alcohol solvents (ethanol, benzyl alcohol, propylene glycol, etc.), while phosphatidylglycerol (DPPG), phosphatidylethanolamine (DPPE) , phosphatidic acid (DPPA) can not be dissolved in the above solvents.
Embodiment 2
[0108] Embodiment 2 Composition ratio research
[0109] (1) Form test of composition at room temperature
[0110] The compositions were prepared according to each ingredient and ingredient ratio shown in Table 2-1 below. The solvent phospholipid, oil and solvent were mixed, heated and stirred until a transparent and homogeneous solution was formed, cooled to room temperature, and the physical form was investigated. At the same time, the room temperature form of the unsaturated phospholipid composition was used as a comparative study.
[0111] Table 2-1 Room temperature form of compositions under different ratios
[0112]
[0113] In this example, the formulations of compositions containing 0.5-30% (w / w) saturated phospholipids (HSPC, DPPC, DSPC and DMPC) and 0-50% (w / w) solvent at room temperature were investigated, and the results are shown in Table 2 As shown in -1, the room temperature form of the composition of the present invention is related to the amount of satura...
Embodiment 3
[0122] (1) Effects of different types of phospholipids on the phase transition of the composition
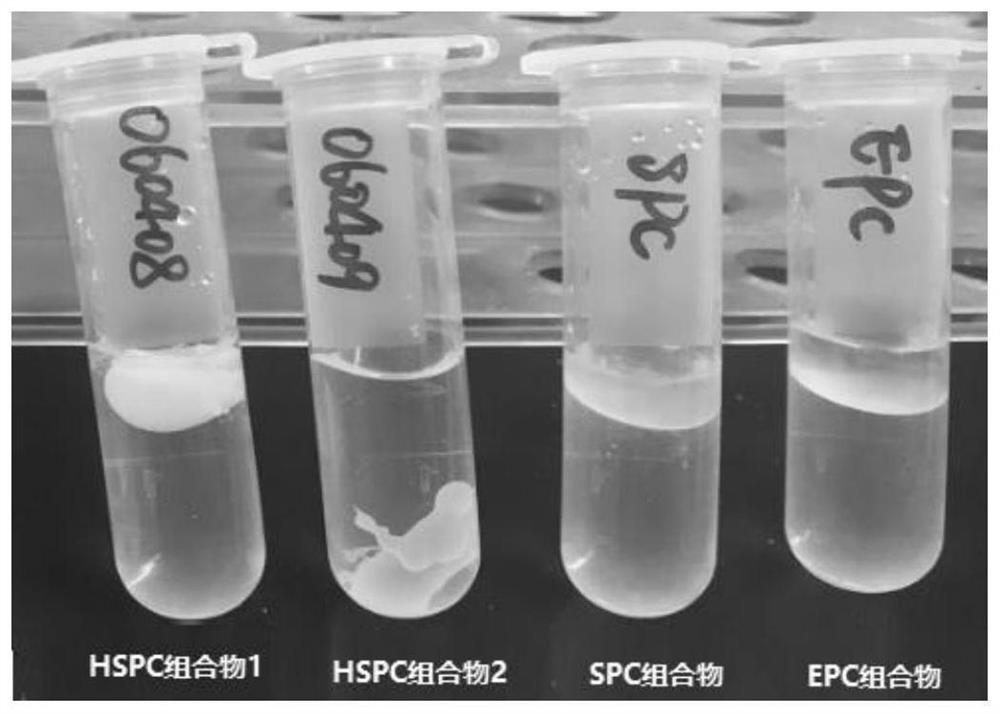
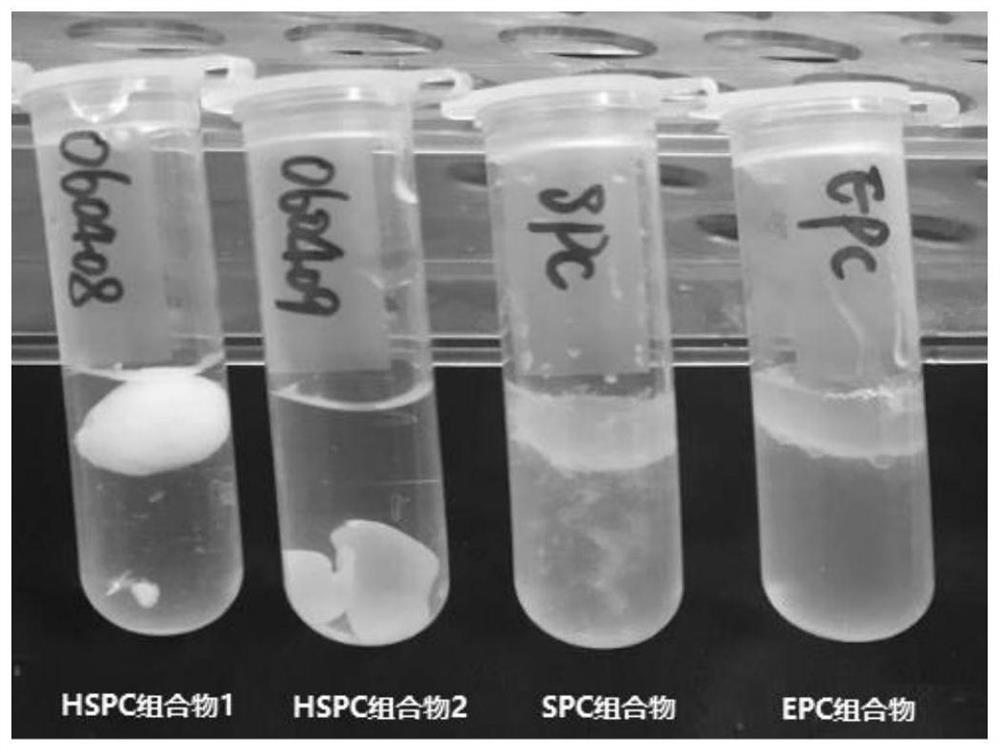
[0123] Pharmaceutical compositions containing saturated phospholipids (composition 16), without phospholipids (pure castor oil group) and containing unsaturated phospholipids were prepared according to Table 3-1. Meloxicam was dissolved in N-methylpyrrolidone to prepare a concentrated solution of 50 mg / g, ropivacaine, soy lecithin or dipalmitoyl phosphatidylcholine were added to liquid oil, solvent and meloxicam at 50°C Kang solution, heating and stirring until a transparent and homogeneous solution was formed, and cooled to room temperature. The composition was slowly injected into water with a syringe, and the morphological changes of different compositions in water were observed. see the results Picture 1-1 .
[0124] Table 3-1 Different compositions
[0125]
[0126] The above experiments respectively investigated the morphological changes in water containing saturate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com