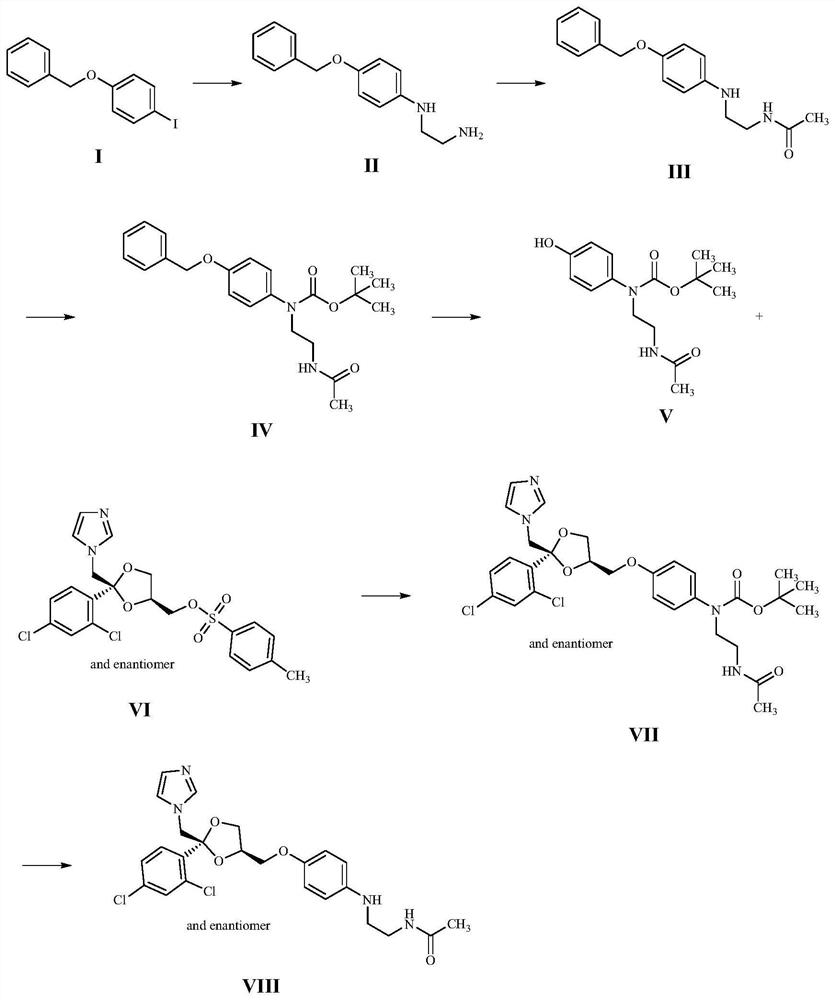
Synthesis method of ketoconazole metabolite
A synthesis method and metabolite technology, applied in organic chemistry and other directions, to achieve the effect of reasonable process design, easy availability of raw materials and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of synthetic method of ketoconazole metabolite, comprises the following steps:
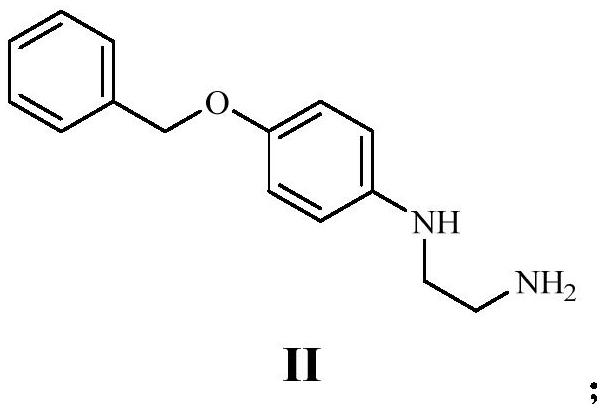
[0046] (1) Dissolve 34.00g of 1-benzyloxy-4-iodobenzene (compound I) in N,N-dimethylformamide, add 11.21g of ethylenediamine, 6.12g of sodium hydroxide and 0.51g of bromine Cuprous chloride was stirred and reacted at room temperature for 5 hours. After the reaction was complete, a brownish-black turbid liquid was obtained, which was diluted with water and extracted with ethyl acetate to obtain 26.00 g of gray solid compound II with a yield of 97.87%.
[0047] (2) Dissolve 26.00 g of compound II in tetrahydrofuran, add 15.30 g of acetic anhydride, and stir at room temperature for 1 hour. After the reaction is complete, a gray cloudy liquid is obtained. After concentration, 26.55 g of gray solid compound III is purified by column chromatography, with a yield of 87.02%. .
[0048] (3) Dissolve 5.00 g of compound III in 1,4-dioxane, add 5.21 g of di-tert-butyl dicarbonate and 3.12 g of ...
Embodiment 2
[0053] A kind of synthetic research of ketoconazole metabolite, comprises the following steps:
[0054] (1) Dissolve 20.00g of 1-benzyloxy-4-iodobenzene (compound I) in tetrahydrofuran, add 11.48g of ethylenediamine, 5.06g of sodium hydroxide and 0.82g of ferrous chloride, and stir at room temperature for 5 After 1 hour, the reaction was complete to obtain a brown-black turbid liquid, which was diluted with water and extracted with ethyl acetate to obtain 15.12 g of gray solid compound II, with a yield of 96.76%.
[0055] (2) Dissolve 15.12g of Compound II in tetrahydrofuran, add 4.76g of acetyl chloride, and stir at room temperature for 1 hour. After the reaction is complete, a gray cloudy liquid is obtained. After concentration, 17.34g of gray solid Compound III is purified by column chromatography, with a yield of 97.73%. .
[0056] (3) Dissolve 5.00 g of compound III in dichloromethane, add 4.22 g of di-tert-butyl dicarbonate and 1.62 g of sodium bicarbonate, stir and rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com