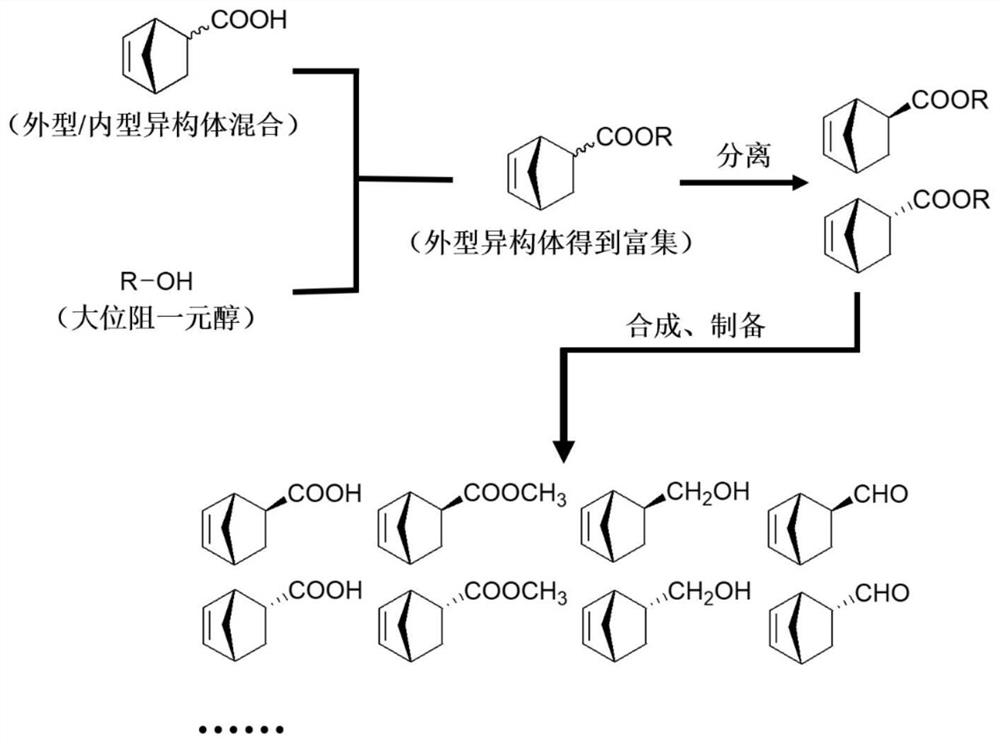
Method for preparing single-configuration C-2-position-monosubstituted norbornene derivative
A technology of norbornene and C-2, which is applied in the field of preparing single-configuration C-2-position monosubstituted norbornene derivatives, can solve the problems of difficult preparation of exoisomers and difficult separation conditions, and achieve raw material The effect of easy availability and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1, Preparation and separation of exo / endo-5-norbornene-2-carboxylate tert-butyl ester
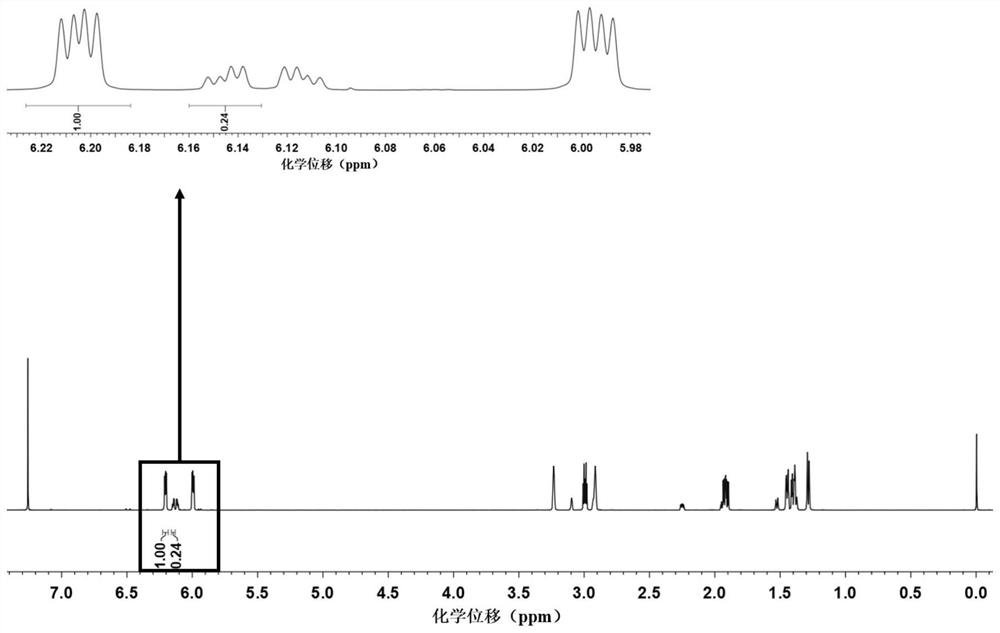
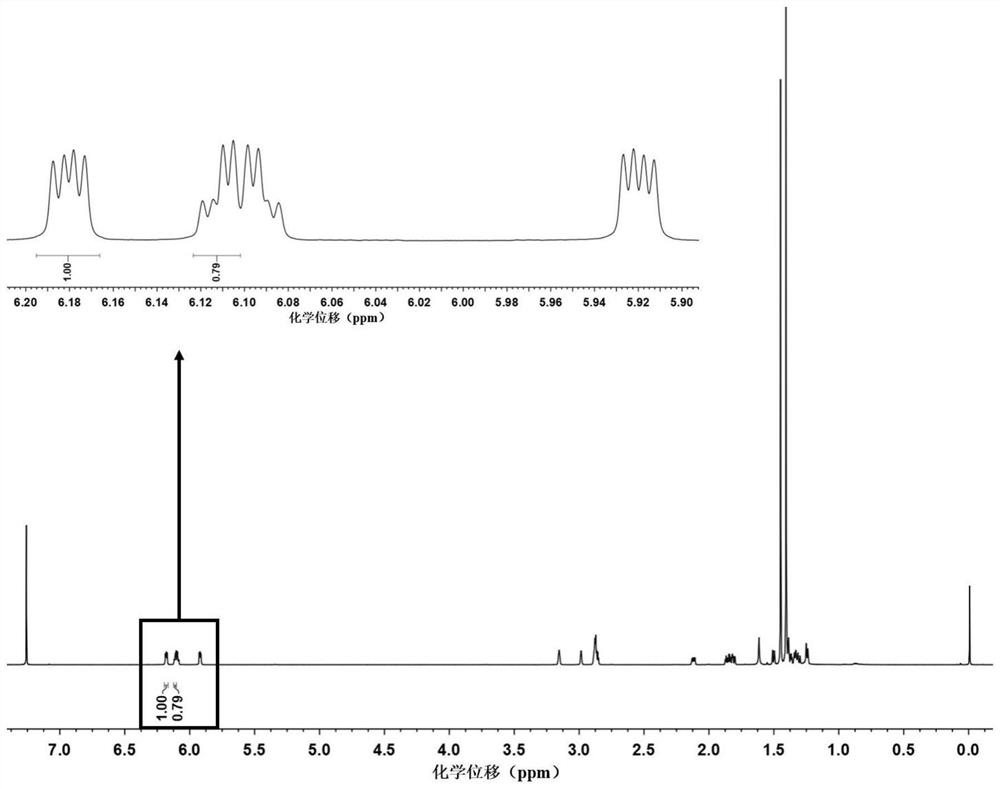
[0028] Add 5-norbornene-2-carboxylic acid (5.0 g, 36.2 mmol, its 1 H NMR spectrum shows that its exo / endo isomer molar ratio is about 19:81). Then add 5mL tert-butanol (4.0g, 54.3mmol) and 4-dimethylaminopyridine (0.4g, 3.6mmol), the above system is dissolved with 20mL dichloromethane, then add 15mL dichloromethane dissolved 1 -Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (8.4g, 54.3mmol), stirred at room temperature for 36h. After the reaction was completed, the product was processed by extraction to obtain the mixed 5-norbornene-2 tert-butyl carboxylate (NMR results showed that its exo / endo isomer ratio increased to 44:56). Then, the mixed solution of 5-norbornene-2-carboxylate tert-butyl 5-norbornene-2-carboxylate with exo / endo isomers as filler, ethyl acetate and sherwood oil as eluent, was separated by column chromatography , the exo-tert-butyl 5-norborn...
Embodiment 2
[0029] Example 2, Preparation and separation of exo / endo-5-norbornene-2-carboxylate isopropyl ester
[0030] Add 5-norbornene-2-carboxylic acid (5.0 g, 36.2 mmol, its 1The HNMR spectrum shows that its exo / endo molar ratio is about 20:80). Then add 4mL of isopropanol (3.3g, 54.3mmol) and N-hydroxysuccinimide (0.4g, 3.6mmol), with 18mL of dichloromethane to dissolve the above system, then add 15mL of dichloromethane dissolved in the system N,N'-dicyclohexylated carbodiimide (11.2g, 54.3mmol), stirred at room temperature for 36h. After the reaction was completed, the product was processed by extraction to obtain the mixed 5-norbornene-2-carboxylic acid isopropyl ester of exo / endo isomers (NMR results showed that its exo / endo isomer ratio improved to 49:51). Then, the isopropyl 5-norbornene-2-carboxylate mixed with exo / endo isomers was separated and purified by fractional distillation to obtain exo-5-norbornene- 2-Isopropyl carboxylate and endo-5-norbornene-2-carboxylate isopr...
Embodiment 3
[0031] Example 3, Preparation of exo / endo-5-norbornene-2-carboxylic acid pentafluorophenyl ester
[0032] Add 5-norbornene-2-carboxylic acid (5.0 g, 36.2 mmol, its 1 H NMR spectrum shows that its exo / endo ratio is about 23:77). Then add pentafluorophenol (10.0g, 54.3mmol) and 4-dimethylaminopyridine (0.4g, 3.6mmol), the above system is dissolved with 30mL of dichloromethane, then 1- Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (8.4g, 54.3mmol), stirred at room temperature for 48h. After the reaction was completed, the product was processed by extraction to obtain the mixed 5-norbornene-2-carboxylic acid pentafluorophenyl ester of exo / endo isomers (NMR results showed that the ratio of exo / endo isomers improved to 57:43). Then, the mixed solution of 5-norbornene-2-carboxylate pentafluorophenyl ester of exo / endo isomer is carried out column chromatography with silica gel powder as filler, dichloromethane and sherwood oil as eluent Separation, the exo-pentafluorophe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com