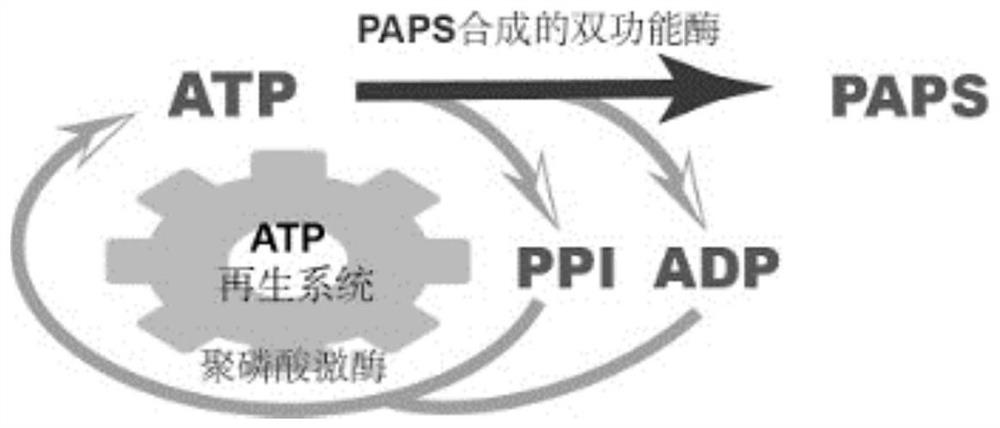
Efficient catalytic synthesis method of PAPS based on construction of ATP regeneration system
A catalyst and sulfurylase technology, applied in the field of bioengineering, can solve the problem that by-products cannot be fully utilized, and achieve the effects of simple preparation steps, good stability and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: Obtaining of polyphosphate kinase
[0067] (1) Obtain the Gene ID: 1020661 of the polyphosphokinase from Corynebacterium glutamicum on the NCBI website, obtain the nucleotide sequence through codon optimization, use polymerase chain reaction to amplify the target gene, and connect it to pRSFDuet-1 On the vector, a recombinant plasmid was obtained, and the verified correct recombinant plasmid was transferred into E.coli BL21(DE3) to obtain a transformant. The transformant was cultured and sequenced for verification, and the verified correct transformant was recorded as strain E.coli GluPPK.
[0068] (2) Find the Gene ID of the polyphosphokinase derived from Mycobacterium tuberculosis: 888760, obtain the nucleotide sequence through codon optimization, and use a method similar to step (1) to amplify and connect the target gene to the vector pET32a (+), and the verified correct recombinant plasmid was transferred into E.coli BL21(DE3) to obtain a transformant,...
Embodiment 2
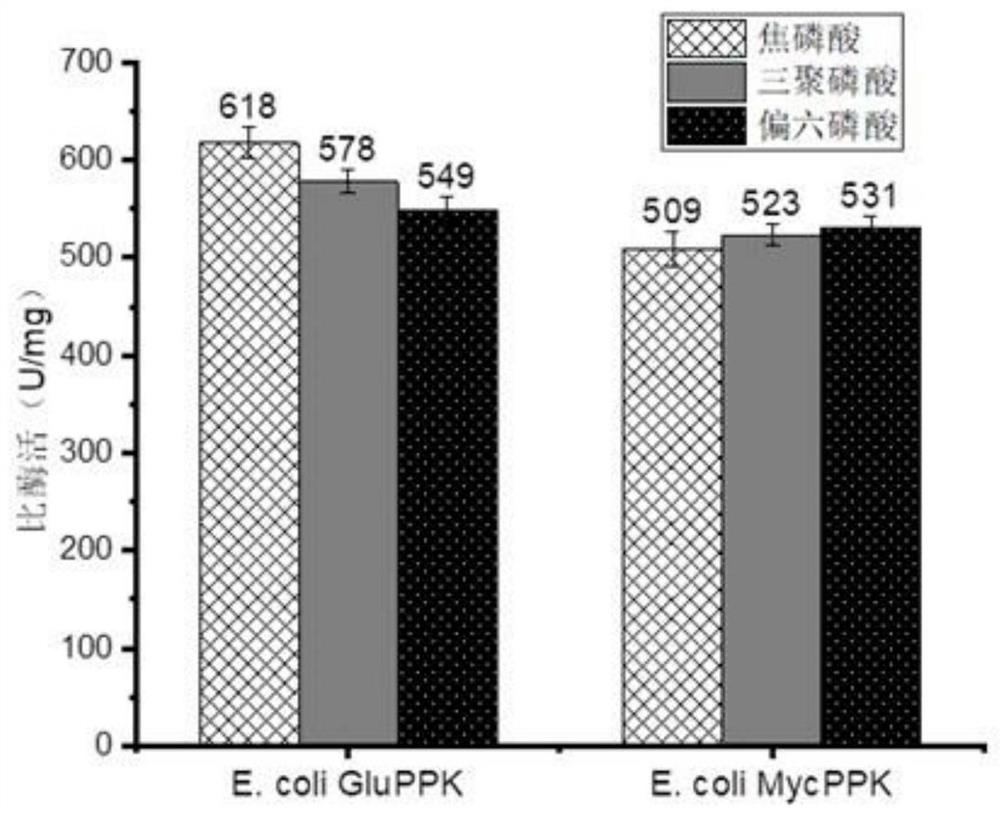
[0072] Embodiment 2: Purification and enzymatic activity comparison of two kinds of polyphosphokinases
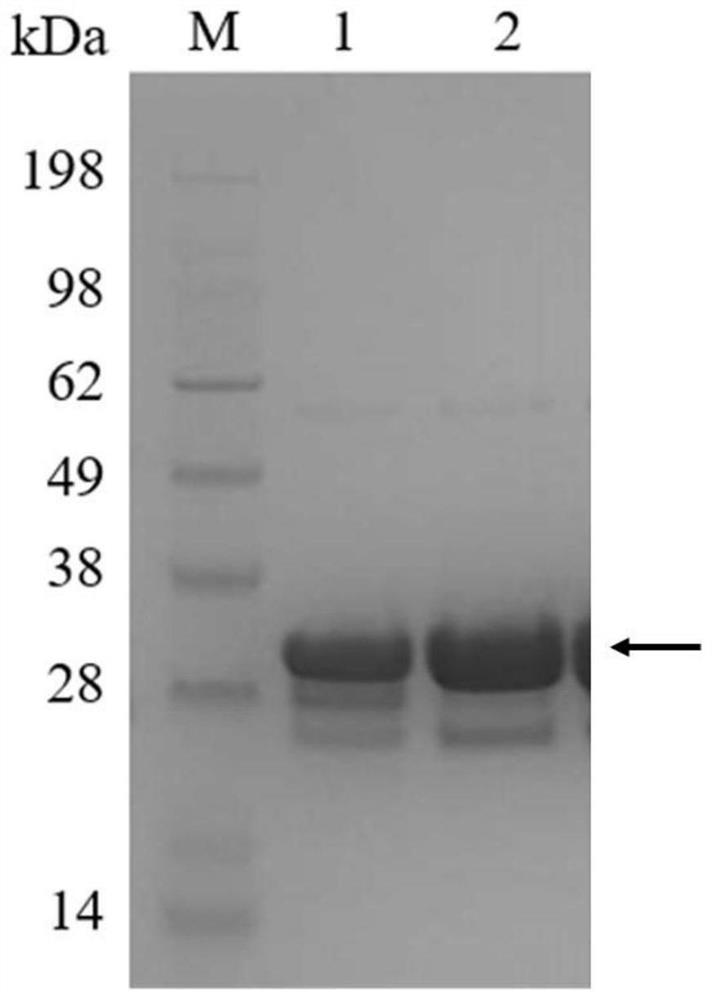
[0073] After the recombinant strain obtained in Example 1 was cultured in LB medium for 6-20 hours, the collected bacteria were ultrasonically disrupted and then centrifuged at high speed to remove cell debris. The supernatant was filtered through a 0.22 μm aqueous membrane, and the Ni-NTA affinity layer was used to analysis to purify the protein of interest. After equilibrating the column with solution A, load the crude enzyme solution, then use solution A to balance the chromatography column, and then wash the column with different concentrations of solution B and collect the washing solution, and use SDS-PAGE to verify the purified components ( figure 2 ), and the purest fraction was desalted with a PD-10 desalting column, and a low-salt buffer (10mM Tris-HCl, 0.1M NaCl; pH 6.0) was used for desalting, and the purified desalted protein was collected.
[0074] Solution ...
Embodiment 3
[0077] Example 3: Expression, purification and enzyme activity assay of a synthetic bifunctional enzyme
[0078] Construction of a bifunctional enzyme: ATP sulfurylase (Gene ID 853466) derived from Saccharomyces cerevisiae and APS kinase derived from Escherichia coli (Gen Bank No. M74586.1) were fused into a fragment according to genetic manipulation means (the sequence does not affect its expression), add different fusion linker sequences (linker) to the joint part respectively, and fuse them into one fragment, so that both enzymes maintain a certain spatial position, and the catalysis is more orderly. Specifically, the previous gene's Remove the stop codon, connect directly with the linker, and then connect with the start codon of another gene. The linker sequence is: (GGGGS) 6 , the fusion fragment was connected to the plasmid pET28a(+), and transformed into E.coli BL21(DE3) to obtain a transformant, the transformant was cultured and sequenced for verification, and the veri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com