Urine keratin, II-type cytoskeleton 1 and application of polypeptide fragment thereof in allergic diseases
A technology of allergic diseases and cytoskeleton, which is applied in disease diagnosis, material inspection products, measuring devices, etc., can solve the problems of poor patient compliance, increased systemic allergic symptoms, and susceptibility to drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Urine Specimen Collection and Processing
[0023] A total of 60 outpatients diagnosed with allergic diseases in Beijing Shijitan Hospital Affiliated to Capital Medical University were collected as the allergic disease (allergic disease) group, aged 23-58 years. A total of 60 healthy persons who underwent physical examination at the Physical Examination Center of Beijing Shijitan Hospital Affiliated to Capital Medical University were collected as the control group. After the urine was collected, it was centrifuged at 400×g for 5 minutes, the supernatant was taken, aliquoted, and frozen at -80°C. A comparison of the clinical characteristics of these study subjects is shown in Table 1.
[0024] Table 1 Comparison of general clinical data between the allergic disease (allergic disease) group and the control group.
[0025]
Embodiment 2
[0026] Example 2 Magnetic bead purification and screening of urine peptides
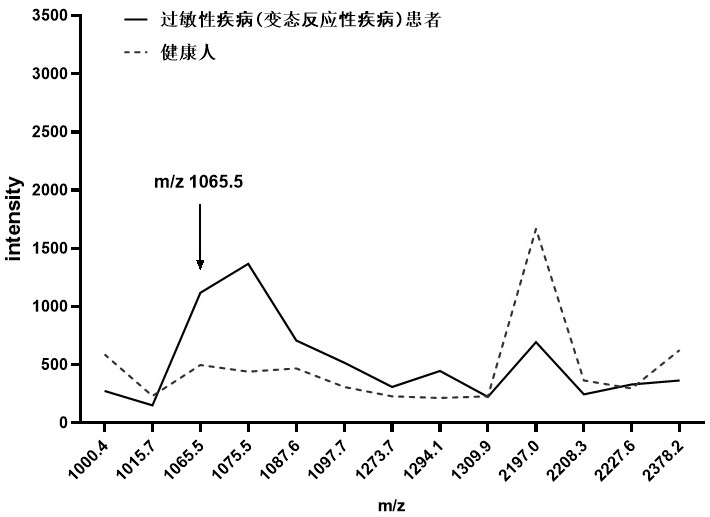
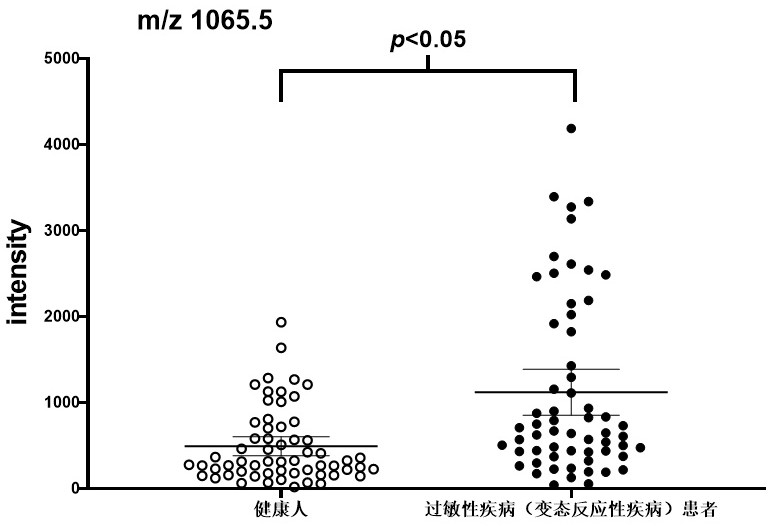
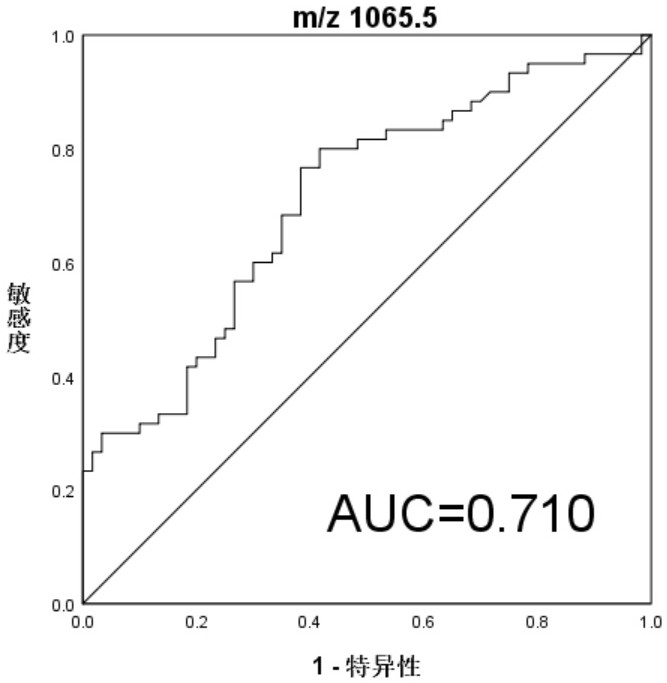
[0027]Weak cation-exchange magnetic beads were used to enrich the peptides in urine, and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) was used to analyze the peptide spectrum in urine. Mix 1 μl of eluate with 10 μl of matrix (0.3 % α-cyano-4-hydroxycinnamic acid, HCCA), take 1 μl to spot on the Anchorchip target plate, and dry at room temperature. The specimens were ionized by irradiation with a nitrogen laser, and calibrated before the specimens were subjected to further mass spectrometric analysis. After calibrating the instrument with a standard, test the sample, collect data in the range of 1-10kDa, and obtain a mass spectrum composed of protein peaks with different mass-to-charge ratios. For each MALDI target, a total of 400 laser shots were made. 8 different positions of each target point were irradiated 50 times, and the average value represe...
Embodiment 3
[0028] Example 3 Identification and Analysis of Differential Peptides
[0029] Take 20 ul from the sample tube for LC-MS / MS analysis. EASY-nLC1000 HPLC system was used for separation. Liquid phase A was 0.1% formic acid in acetonitrile (2% acetonitrile), and liquid phase B was 0.1% formic acid in acetonitrile (98% acetonitrile). The chromatographic spray needle is PN: FS360-20-10-N-20-C12. Samples were separated by capillary high-performance liquid chromatography and analyzed by a Q-Exactive mass spectrometer (Thermo). The ion source voltage is 3.5kv, the analysis time is 120min, and the precursor ion scanning range is 300-2000m / z. Mass-to-charge ratios of peptides and peptide fragments were collected as follows: 20 fragments were collected after each full scan (MS2scan, HCD). At M / Z200, MS1 has a resolution of 70,000 and MS2 has a resolution of 17,500. The data analysis software Peaks8.5 (Bioinformatics Solutions Inc.) was used for retrieval. The search database was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com