Non-aqueous slow-release drug delivery system
A drug delivery system and slow-release technology, applied in the field of pharmacy, can solve the problems of low drug availability, low bioavailability, and poor patient tolerance, achieve good application prospects, improve drug utilization, and reduce drug waste Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Accurately weigh 300mg of lidocaine hydrochloride, 1.0g of benzyl alcohol, 2.0g of soybean lecithin S100 and 6.2g of ethyl oleate into a pre-weighed round bottom flask, add excess ethanol, and ultrasonically dissolve the contents completely Connect the round bottom flask to a suitable rotary evaporator, evaporate under reduced pressure until the weight change of the round bottom flask shows that absolute ethanol has been removed. The flask was cooled to room temperature, 0.5 g of fish oil was added and mixed, and the obtained contents were transferred to a glass bottle to obtain Composition 1.
Embodiment 2
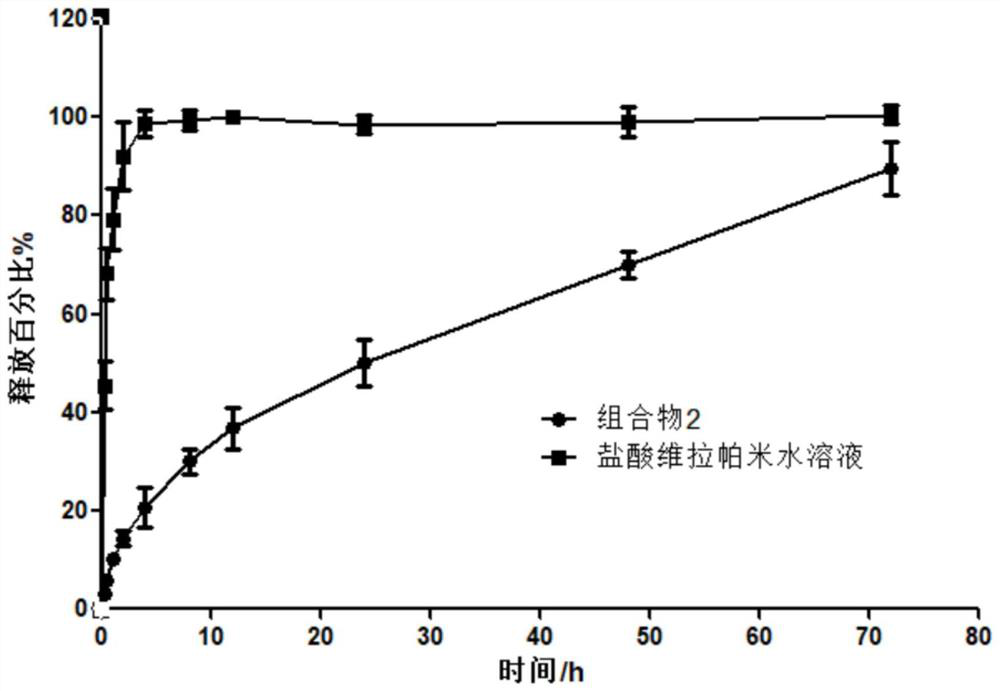
[0092] Accurately weigh 200mg of verapamil hydrochloride, 1.0g of egg yolk lecithin E80, 8.1g of soybean oil and 0.2g of linseed oil into a pre-weighed round bottom flask, add excess ethanol, and ultrasonically dissolve the contents completely Connect the round-bottomed flask to a suitable rotary evaporator, and evaporate under reduced pressure until the weight change of the round-bottomed flask shows that absolute ethanol has been removed. The flask is cooled to room temperature, and 0.5g of absolute ethanol is added and mixed to obtain a combination Object 2.
Embodiment 3
[0094] Accurately weigh 500mg of promethazine free base, 2.0g of benzyl alcohol, 2.5g of egg yolk lecithin E80 and 4.0g of castor oil into a pre-weighed round bottom flask, add excess ethanol, and ultrasonically dissolve the contents completely; Connect the round bottom flask to a suitable rotary evaporator, and evaporate under reduced pressure until the weight change of the round bottom flask indicates that absolute ethanol has been removed, then cool the flask to room temperature, add 1.0 g of fish oil and mix well to obtain composition 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com