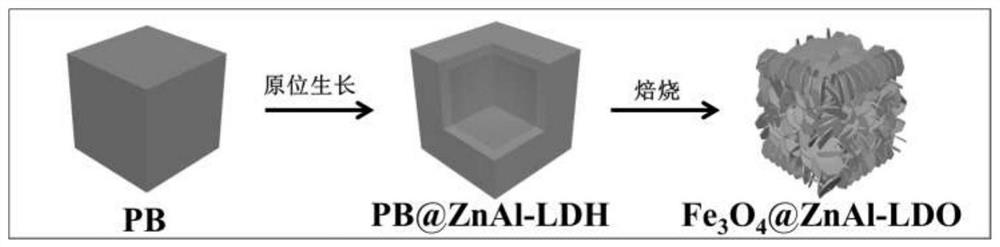
Preparation method of magnetic adsorption material based on Prussian blue and hydrotalcite
A magnetic adsorption material, Prussian blue technology, applied in chemical instruments and methods, other chemical processes, alkali metal compounds, etc., can solve problems such as difficult recovery and poor recycling effect, reduce costs, and solve secondary treatment of water bodies The problem, the effect of the simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
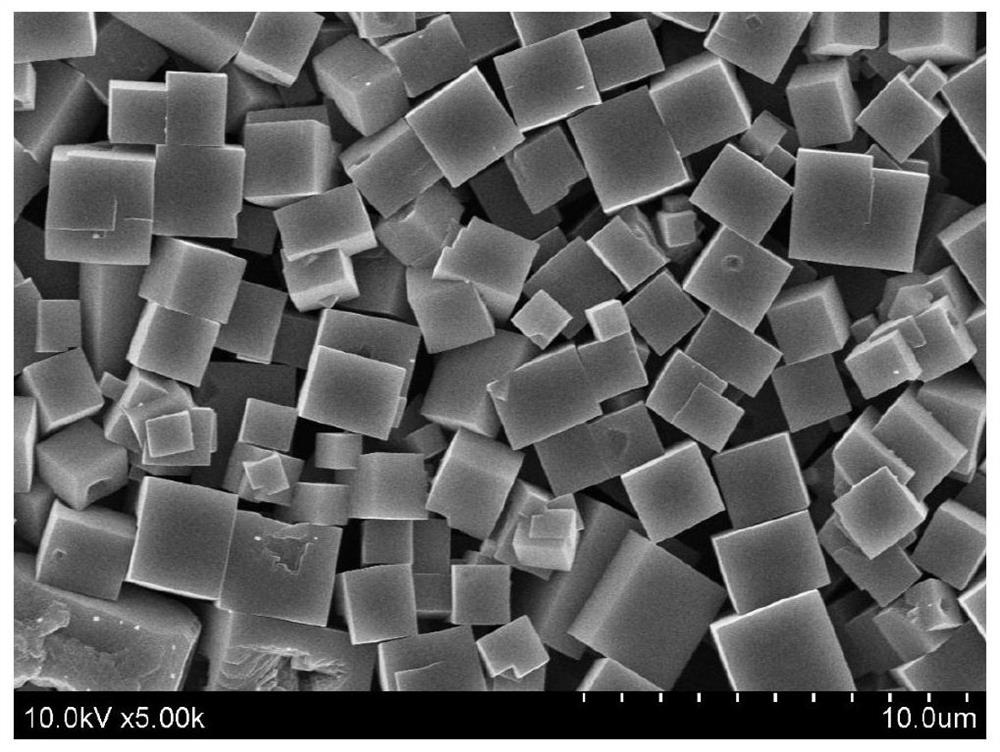
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of ZnAl-LDH on the surface of PB, forming Fe after calcination 3 o 4 @ZnAl-LDO magnetic composite material
[0028] The preparation process comprises the following steps:
[0029] a: Disperse 0.3 g of PB in 70 mL of aqueous solution dissolved with zinc chloride, aluminum nitrate, urea, and citric acid, in which the concentration of urea is 0.1 mol L –1 , the concentration of citric acid is 0.02 mol L –1 , the concentration of zinc ions is 0.03 mol L –1 , the concentration of aluminum ions is 0.01 mol L –1 ;
[0030] b: Put in a 100 mL reactor, react at 100°C for 24 h, wash and dry the obtained solid product;
[0031] c: Put the product obtained in step b into a tube furnace with a heating rate of 5°C min –1 , calcined at 400°C for 5 h under nitrogen atmosphere to obtain Fe 3 o 4 @ZnAl-LDO magnetic composite.
Embodiment 2
[0032] Example 2 Synthesis of CoAl-LDH on the surface of PB, forming Fe after calcination 3 o 4 @CoAl-LDO magnetic composite
[0033] The preparation process comprises the following steps:
[0034] a: Take 0.4 g PB and disperse it in 100 mL aqueous solution dissolved with cobalt chloride, aluminum nitrate, urea, and citric acid, and the concentration of urea is 0.4 mol L –1, the concentration of citric acid is 0.08 mo L –1 , the concentration of cobalt ions is 0.02 mo L –1 , the concentration of aluminum ions is 0.08 mol L –1 ;
[0035] b: Put in a 200 mL reactor, react at 110°C for 20 h, wash and dry the obtained solid product;
[0036] c: Put the product obtained in step b into a tube furnace with a heating rate of 2°C min –1 , calcined at 500°C for 5 h under helium atmosphere to obtain Fe 3 o 4 @CoAl-LDO Magnetic Composite.
Embodiment 3
[0037] Example 3 Synthesis of MgAl-LDH on the surface of PB, forming Fe after calcination 3 o 4 @MgAl-LDO magnetic composite material
[0038] The preparation process comprises the following steps:
[0039] a: Disperse 1.0 g of PB in 300 mL of aqueous solution dissolved in magnesium chloride, aluminum nitrate, urea, and citric acid, in which the concentration of urea is 0.8 mol L –1 , the concentration of citric acid is 0.1 mol L –1 , the concentration of magnesium ions is 0.05 mol L –1 , the concentration of aluminum ions is 0.03 mol L –1 ;
[0040] b: Put in a 500 mL reactor, react at 110°C for 20 h, wash and dry the obtained solid product;
[0041] c: Put the product obtained in step b into a tube furnace with a heating rate of 2°C min –1 , calcined at 600°C for 4 h under nitrogen atmosphere to obtain Fe 3 o 4 @MgAl-LDO magnetic composites.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com