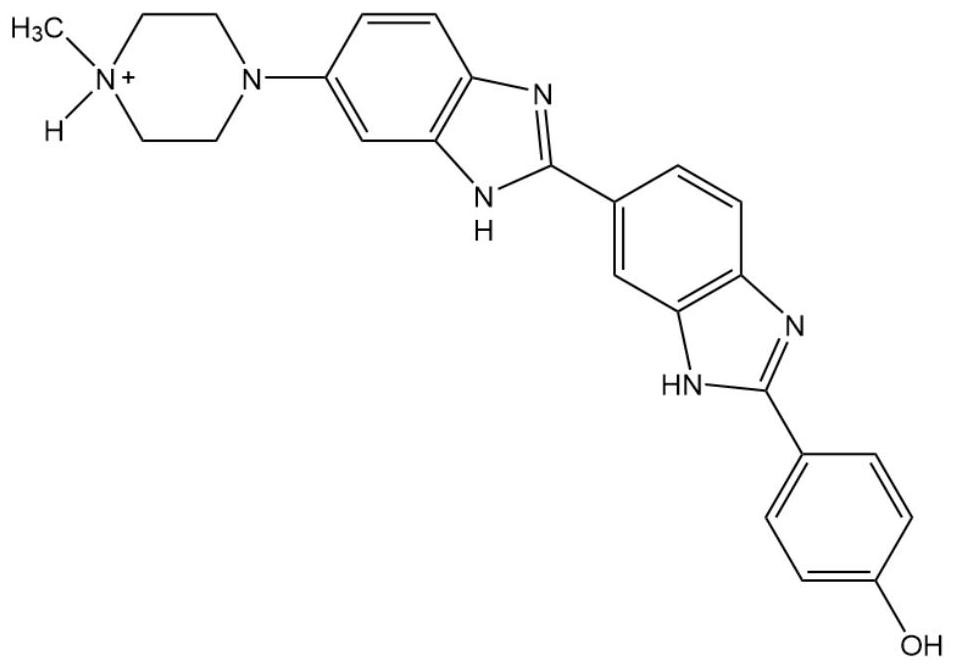
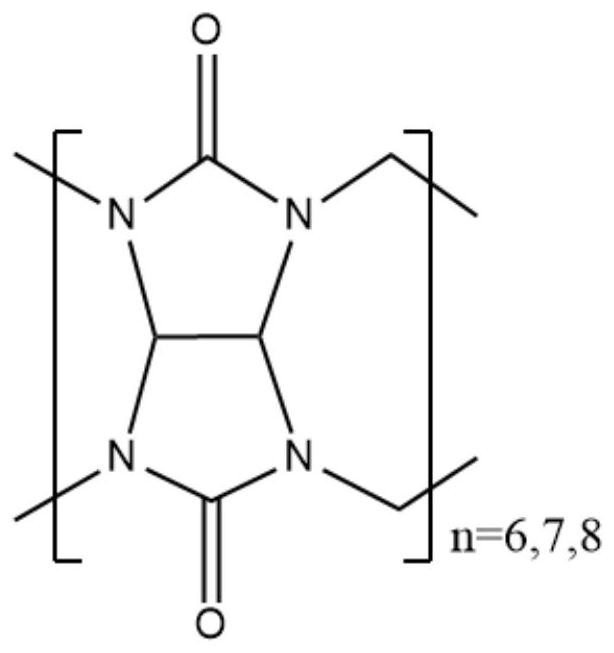
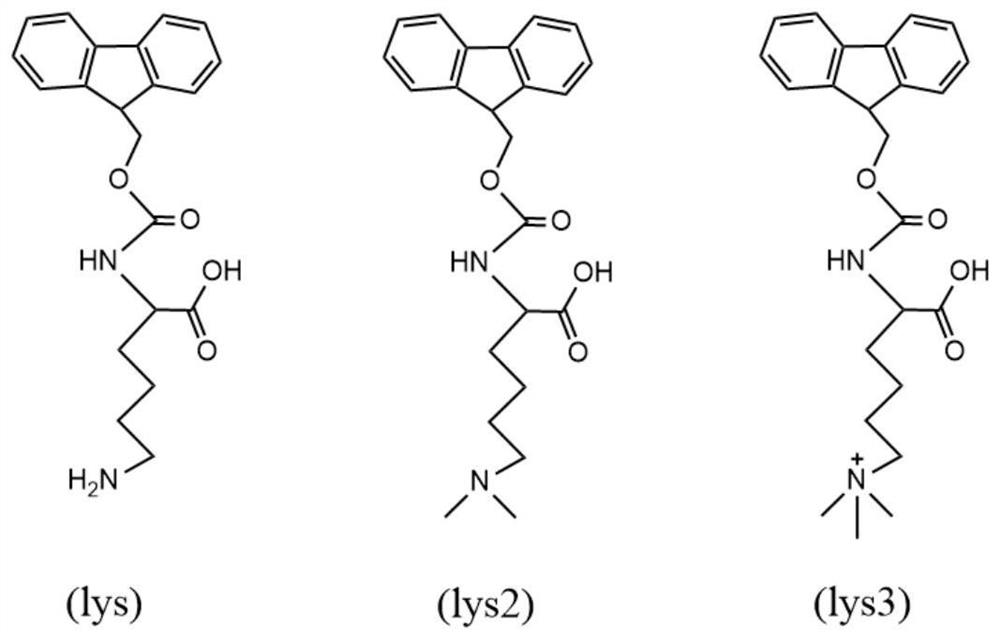
Molecular assembly type fluorescent probe as well as preparation method and application thereof
A fluorescent probe and molecular technology, applied in the field of biochemistry, can solve the problems of affecting the analysis sensitivity, reducing the hydrogen bond force of amino acid residues, and the difficulty in identifying methylated peptides, achieving fast detection speed and high detection accuracy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Preparation method of molecular assembly fluorescent probe
[0050] First, prepare cucurbituril CB[n]:
[0051] Weigh 80.0 g of urea, stir to dissolve it in 250 mL of deionized water, and use concentrated sulfuric acid to adjust the pH value of the solution to 1-2. Under stirring, slowly add 62ml of glyoxal (40% aqueous solution) dropwise to the solution with a dropping funnel, at a rate of about 3 seconds per drop, control the temperature of the oil bath to be lower than 70°C, and raise the temperature to 75°C after the addition is completed About 6h, the reaction is over. The reaction solution was filtered under reduced pressure, washed with water and acetone three times in sequence, and dried in vacuum at 70° C. for 24 hours to obtain 55.8 g of glycoside urea. Weigh 50.0g of glycoside urea and dissolve it in 150mL of concentrated hydrochloric acid. Stir at room temperature and place in an ultrasonic disperser, take it out after 10 minutes to ensure that the glycos...
Embodiment 2
[0053] The application of the molecular assembly fluorescent probe in the recognition of trimethyl-substituted Fmoc-protected lysine, the specific operation steps are as follows:
[0054] 1) First configure 3 parts of fluorescent probe aqueous solution with a concentration of 3.0 μM, measure its emitted fluorescence spectrum at an excitation wavelength of 361 nm, and record the maximum emitted fluorescence intensity I at 400-650 nm for each part 0A , I 0B , I 0C ;
[0055] 2) Add Lys3 dropwise to any of the solutions and mix evenly to ensure that the molar ratio of the added Lys3 to the fluorescent probe is 0.3, measure its emitted fluorescence spectrum at an excitation wavelength of 361nm, and record it at 400 nm. Maximum emission fluorescence intensity I at -650nm 1A ;
[0056] 3) continue to drip Lys3 solution in this solution, the amount of dripping is identical with step 2), same record its maximum emission fluorescence intensity I at 400-650nm place 2A , repeated n ...
Embodiment 3-4
[0059] According to Example 2, the guest molecule Lys3 in step 2) was replaced by Lys2 and Lys respectively, and the fluorescence spectrum was measured after adding Lys2 and Lys in different molar ratios to the fluorescent probe while other conditions remained unchanged. The fluorescence spectrum of Lys is as follows Figure 6 shown. The obtained fluorescence response curve is as follows Figure 7 shown. Obviously, the variation trend of Lys3 curve is the largest, by calculation, I nA / I 0A = 0.44. Therefore, the trimethyl-substituted Fmoc-protected lysine can be accurately and effectively identified.
[0060] It can be understood that the guest molecule in Example 2, 3 or 4 is replaced by an unknown type of lysine, and its I n / I 0 value, if the value is less than or equal to 0.55, it can be judged that Lys3 is included in the solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com