Saccharomyces cerevisiae expressed long-acting recombinant fibronectin and application thereof in cosmetics
A technology for fibronectin and Saccharomyces cerevisiae, applied in the emerging biomedical field, can solve the problems of low feasibility of full sequence expression, large molecular weight of natural FN, and low biological activity of FN protein.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
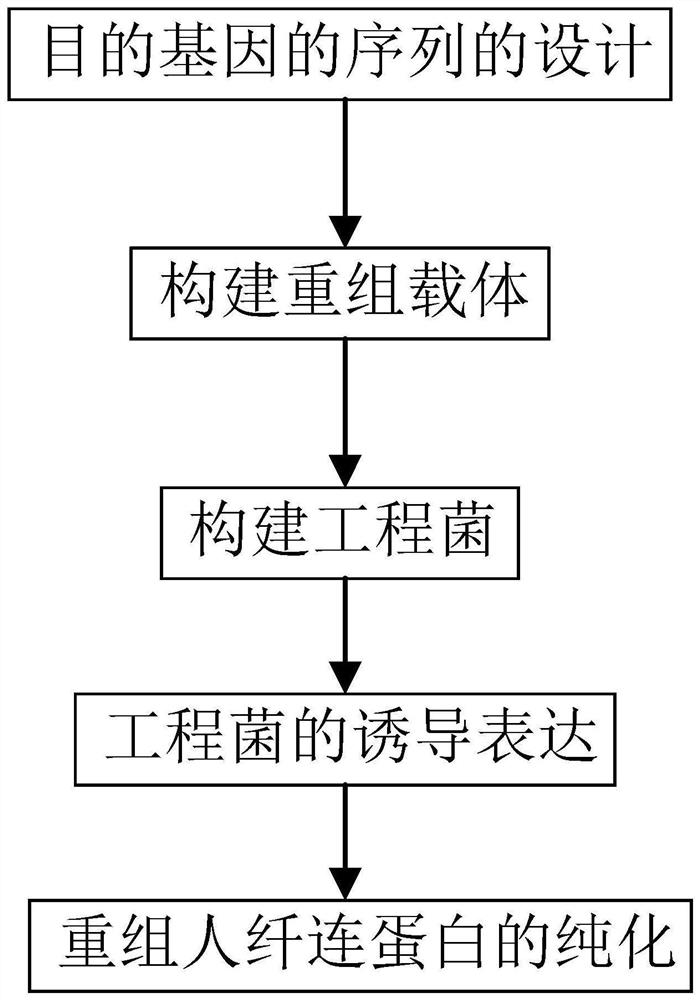
[0062] This example discloses a long-acting recombinant fibronectin expressed by Saccharomyces cerevisiae. It overcomes the complexity and high cost of the natural purification process of FN; the large molecular weight of natural FN and the low feasibility of full sequence expression limit its popularization and application; the existing prokaryotic expression system is easy to form inclusion bodies, and the obtained FN protein has low biological activity and other defects.
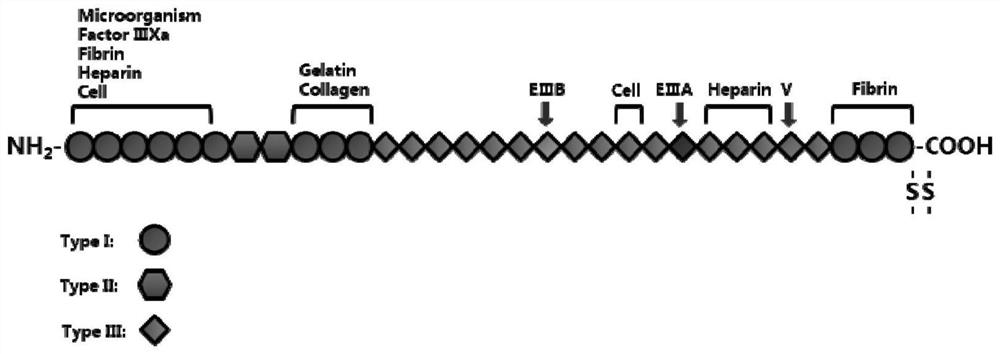
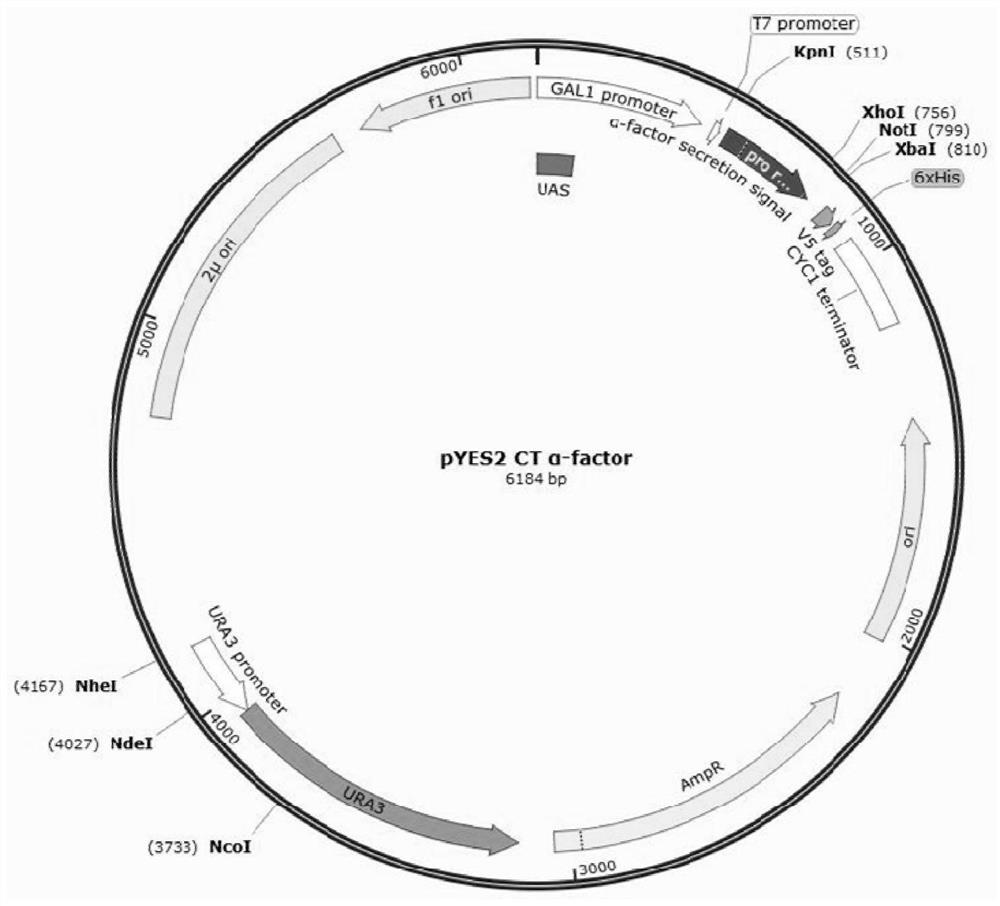
[0063] The long-acting recombinant fibronectin is rFNIII1C-HSS. The nucleotide sequence of the long-acting recombinant fibronectin is shown in Seq ID NO.1, and the amino acid sequence corresponding to the nucleotide sequence of the long-acting recombinant fibronectin is shown in Seq ID NO.2. The nucleotide sequence of long-acting recombinant fibronectin includes the functional fragment of FN, HAS; the functional fragment of FN includes the FNIII1C unit; the FNIII1C unit is a functional fragment of the C-...
Embodiment 2
[0109] This embodiment discloses a method for detecting the adhesion-promoting activity of bovine kidney cells expressed by Saccharomyces cerevisiae and expressing long-acting recombinant fibronectin. Used to verify the attachment activity of the long-acting recombinant fibronectin FNIII1C-HSS promoting bovine kidney cells (MDBK) as in Example 1. The detection method comprises reagent preparation and cell growth promoting activity determination.
[0110] Reagents include: complete culture solution, serum-free culture solution, digestion solution and PBS buffer.
[0111] The preparation of each cultivation and reagent in the present embodiment specifically includes:
[0112] (1) Complete culture medium: Measure 10ml of fetal bovine serum, 1ml of double antibody, and add 90ml of DMEM culture medium.
[0113] (2) Serum-free culture medium: Measure 1ml of double antibody, add 99ml of 1640 culture medium.
[0114] (3) Digestive fluid: 0.25% trypsin.
[0115] (4) PBS buffer solu...
Embodiment 3
[0124] This example provides an experiment of using rFNIII1C-HSA in repairing skin damage, which is used to verify the effect of rFNIII1C-HSA in repairing skin damage in Example 1. Experiments on mouse models have proved that FNIII1C-HSA can effectively promote skin wound repair and significantly increase the healing rate of injuries. The implementation includes the following steps:
[0125] (1) Test groups
[0126] 4-5 week-old experimental rats were divided into 2 groups, 10 in each group, with a body weight of about 250 g, and marked with ear marks.
[0127] (2) Test treatment
[0128] A punch was used to punch a hole (0.5 cm in diameter) in the back, and a PBS smearing control group and a rFNIII1C-HSS (1 mg / ml) smearing experimental group were respectively set up, and smeared 3 times a day until the wound healed. The two groups of rats were raised in a warm and dry environment, and pictures were taken every day to observe and record the wound healing.
[0129] (3) Expe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com