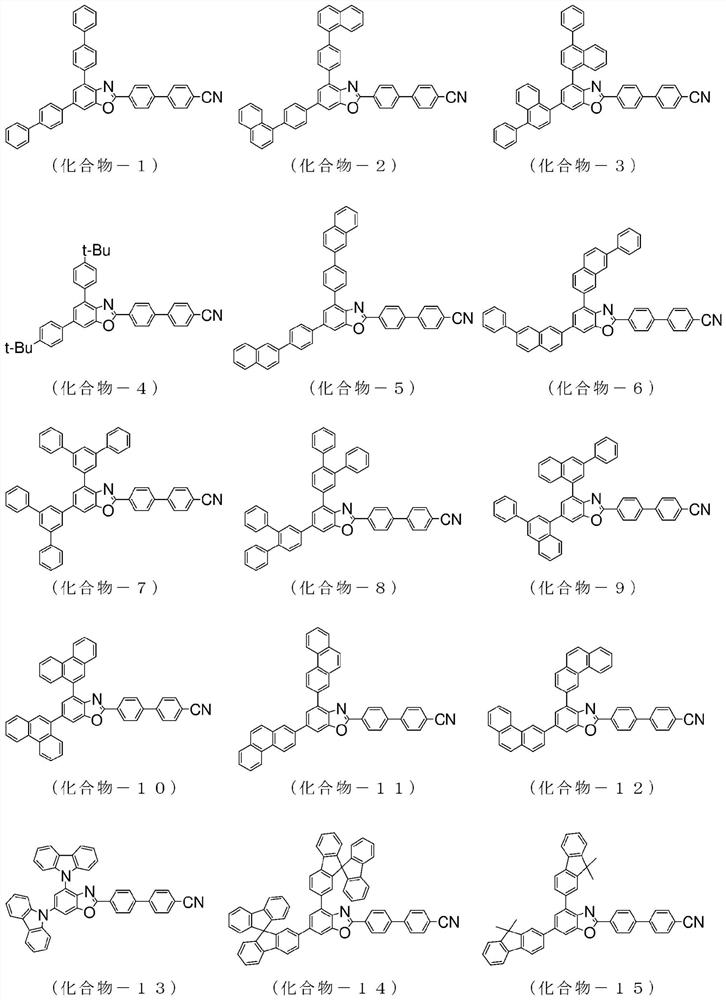
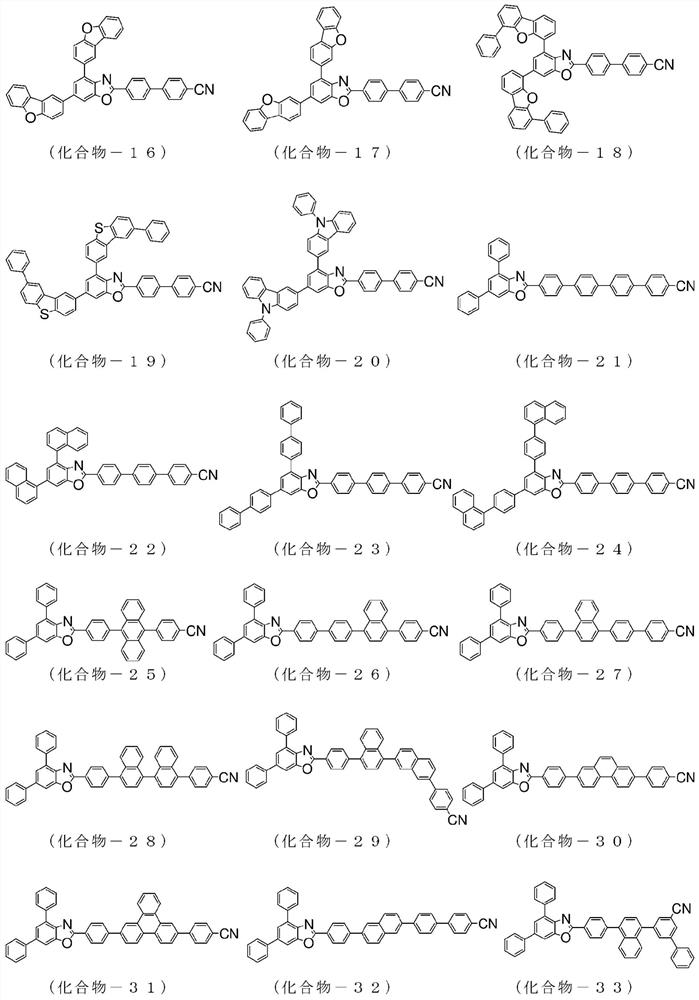
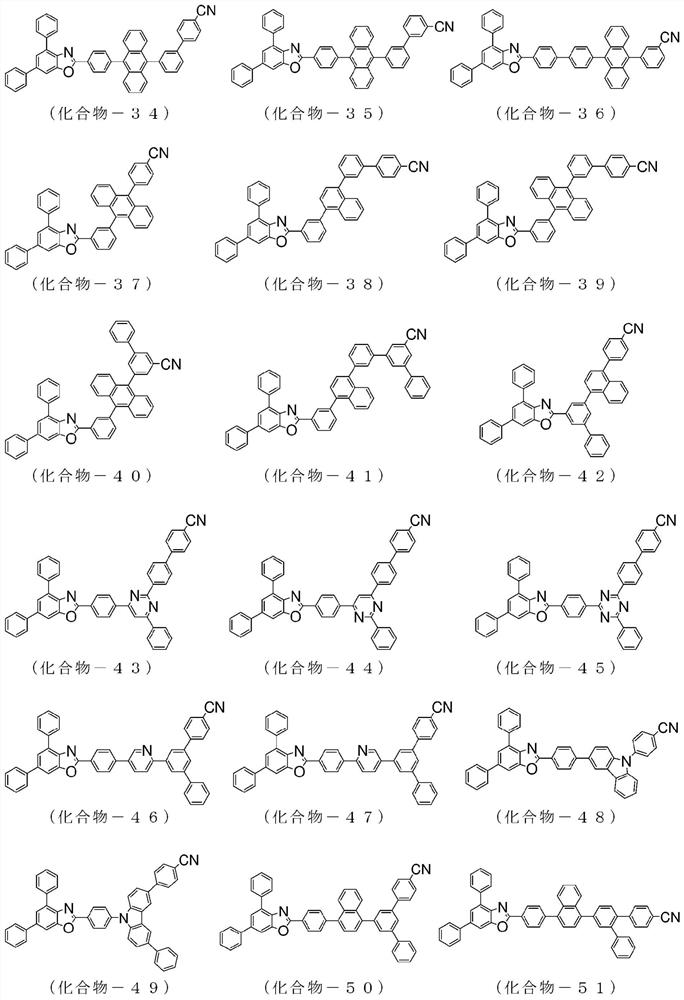
Compound having benzazole ring structure, and organic electroluminescent device
A technology of benzoxazole ring and compound is applied in the fields of compounds with benzoxazole ring structure and organic electroluminescence elements, which can solve the problems of insufficient hole blocking function, lack of film stability, low electron transport, etc. Improved electron transport efficiency, excellent hole blocking ability, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156]
[0157] 2-(4-chlorophenyl)-4,6-bis(4-naphthalen-1-yl-phenyl)-benzoxazole: 8.8g, 4-cyanophenylboronic acid: 3.2g, three (dibenzylideneacetone) dipalladium (0): 0.3g, tricyclohexylphosphine: 0.4g, tripotassium phosphate: 5.4g, in 1,4-dioxane, H 2 O mixed solvent under reflux and stirred overnight. After natural cooling, methanol was added into the system, dispersed, washed and filtered to obtain a crude product. Purify the obtained crude product by crystallization using a mixed solvent of chlorobenzene / acetone to obtain 4,6-bis(4-naphthalene-1-yl-phenyl)-2-(4'-cyano-biphenyl Pale yellow powder of -4-yl)-benzoxazole (compound-2): 7.2 g (yield: 83%).
[0158] [chemical 13]
[0159]
[0160] For the obtained pale yellow powder, NMR was used to identify the structure.
[0161] use 1 H-NMR (CDCl 3 ) detected the following 32 hydrogen signals.
[0162] δ(ppm)=8.52(2H), 8.33(2H), 8.13(1H), 8.05(1H), 8.04(1H), 8.01-7.88(7H), 7.85-7.78(6H), 7.75(2H), 7.70 (2H), 7.65-...
Embodiment 2
[0164]
[0165] 2-(4-chloro-phenyl)-4,6-bis(phenanthren-9-yl)-benzoxazole: 10.0g, 4-cyanophenylboronic acid: 3.0g, tris (Dibenzylideneacetone) dipalladium (0): 0.5g, tricyclohexylphosphine: 0.5g, tripotassium phosphate: 7.3g, in 1,4-dioxane, H 2 O mixed solvent under reflux and stirred overnight. After natural cooling, methanol was added into the system, dispersed, washed and filtered to obtain a crude product. Purify the obtained crude product by recrystallization using a chlorobenzene solvent to obtain 4,6-bis(phenanthrene-9-yl)-2-(4'-cyano-biphenyl-4-yl)-benzo White powder of oxazole (compound-10): 4.0 g (yield: 36%).
[0166] [chemical 14]
[0167]
[0168] For the obtained white powder, the structure was identified using NMR.
[0169] use 1 H-NMR (CDCl 3 ) detected the following 28 hydrogen signals.
[0170] δ(ppm)=8.85(2H), 8.79(2H), 8.35(2H), 8.17(1H), 8.05(1H), 8.04(1H), 7.98(2H), 7.91(2H), 7.80-7.58(12H ), 7.65(2H), 7.59(1H)
Embodiment 3
[0172]
[0173]Charge 4,6-bis(naphthalen-1-yl)-2-(4-chloro-phenyl)-benzoxazole in the reaction vessel: 8.0 g, 4'-(4,4,5,5- Tetramethyl-[1,3,2]dioxaborolan-2-yl)biphenyl-4-carbonitrile: 6.1g, Tris(dibenzylideneacetone)dipalladium(0):0.8g , tricyclohexylphosphine: 0.9g, tripotassium phosphate: 10.6g, in 1,4-dioxane, H 2 O mixed solvent under reflux and stirred overnight. After natural cooling, water was added to the system for dispersion, washing and filtration to obtain a crude product. Purify the obtained crude product by recrystallization using a chlorobenzene solvent to obtain 4,6-bis(naphthalene-1-yl)-2-(4"-cyano-[1,1'; 4',1 ”] White powder of terphenyl-4-yl)-benzoxazole (compound-22): 9.3 g (yield: 90%).
[0174] [chemical 15]
[0175]
[0176] For the obtained white powder, the structure was identified using NMR.
[0177] use 1 H-NMR (CDCl 3 ) detected the following 28 hydrogen signals.
[0178] δ(ppm)=8.36(2H), 8.14(1H), 8.03(1H), 7.98(3H), 7.94(1H), 7.85(1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
| electron work function | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com