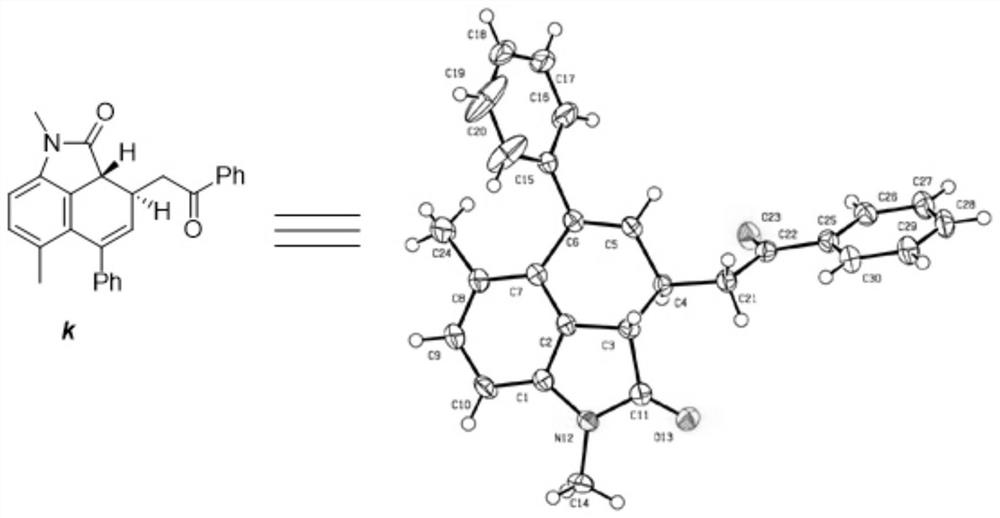
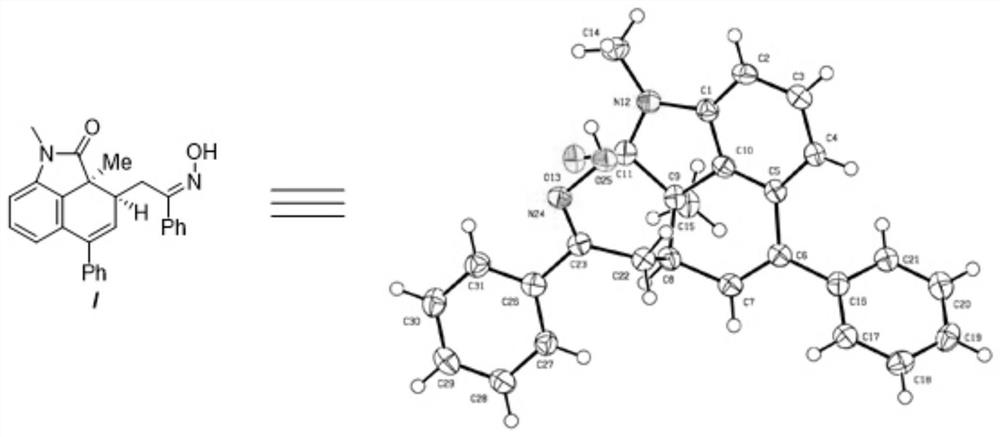
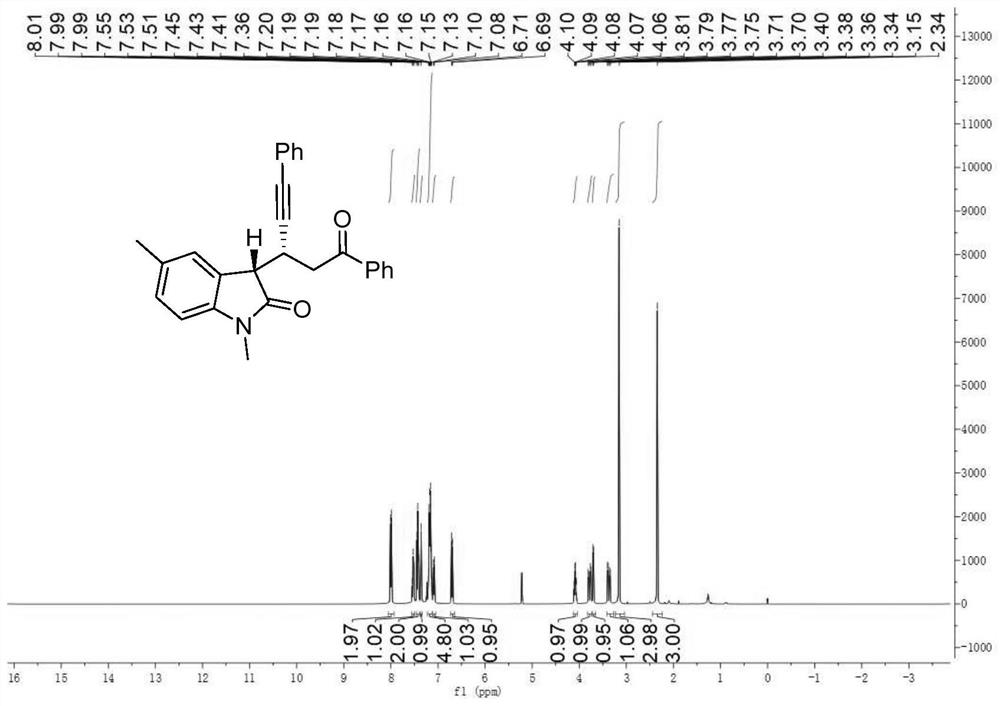
Chiral indolone derivative and synthesis method thereof
A technology of chiral indolinone and synthesis method, which is applied in the field of chiral indolinone derivatives and their synthesis, can solve the problems of limited scope of application of substrates, long synthetic routes, etc., and achieve good application prospects, substrate Wide adaptability and high productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] The synthesis of embodiment 1 compound a
[0097]
[0098] Phenylpropynaldehyde (0.3mmol), p-chloroaniline (0.18mmol), allylpalladium chloride dimer (0.015mmol), (S)-6,6'-bis(triphenylsilyl)spiro Cyclic diphenol phosphate (0.03mmol), Molecular sieves were dissolved in 1.5 mL of anhydrous dichloromethane to prepare mixed solution A; N-methyldiazoacetyl-p-toluidine (0.45 mmol) and β-morpholine styrene (0.36 mmol) were dissolved in 1 mL of anhydrous dichloromethane Prepare mixed solution B; place mixed solution A at 0°C and stir, and add mixed solution B with a syringe pump within 3 hours. After the injection, the reaction system was stirred at 0 °C for 1 h. After the reaction was completed, filter, the filtrate was rotary evaporated to remove the solvent, and then the crude product was purified by column chromatography to obtain pure product a, which was a yellow oily liquid with a yield of 75%, a dr value greater than 20:1, and an ee value of 93%. of the product 1...
Embodiment 2
[0100] The synthesis of embodiment 2 compound b
[0101]
[0102] Phenylpropynaldehyde (0.3mmol), p-chloroaniline (0.18mmol), allylpalladium chloride dimer (0.015mmol), (S)-6,6'-bis(triphenylsilyl)spiro Cyclic diphenol phosphate (0.03mmol), Molecular sieves were dissolved in 1.5 mL of anhydrous dichloromethane to prepare mixed solution A; N-methyldiazoacetyl-p-methoxyaniline (0.45 mmol) and β-morpholine styrene (0.36 mmol) were dissolved in 1 mL of anhydrous Methyl chloride was prepared as mixed solution B; mixed solution A was stirred at 0°C, and mixed solution B was added with a syringe pump within 3 hours. After the injection, the reaction system was stirred at 0 °C for 1 h. After the reaction was completed, filter, the filtrate was rotary evaporated to remove the solvent, and then the crude product was purified by column chromatography to obtain pure product b, which was a yellow oily liquid with a yield of 73%, a dr value greater than 20:1, and an ee value of 90%. ...
Embodiment 3
[0104] The synthesis of embodiment 3 compound c
[0105]
[0106] Phenylpropynaldehyde (0.3mmol), p-chloroaniline (0.18mmol), allylpalladium chloride dimer (0.015mmol), (S)-6,6'-bis(triphenylsilyl)spiro Cyclic diphenol phosphate (0.03mmol), Dissolve molecular sieves in 1.5 mL of anhydrous dichloromethane to prepare mixed solution A; dissolve N-methyldiazoacetanilide (0.45 mmol) and β-morpholine styrene (0.36 mmol) in 1 mL of anhydrous dichloromethane to prepare Mix solution B; place mixed solution A at 0°C and stir, and add mixed solution B with a syringe pump within 3 hours. After the injection, the reaction system was stirred at 0 °C for 1 h. After the reaction was completed, filter, the filtrate was rotary evaporated to remove the solvent, and then the crude product was purified by column chromatography to obtain pure product c, which was a yellow oily liquid with a yield of 67%, a dr value greater than 20:1, and an ee value of 86%. of the product 1 H NMR schematic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com