Alkali metal charge compensation type aluminophosphate red luminescent material as well as preparation method and application thereof
A technology of aluminophosphate and red light emission, which is applied in the direction of light-emitting materials, chemical instruments and methods, circuits, etc., and can solve the problems of short fluorescence life, low quantum efficiency of light emission, poor thermal stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
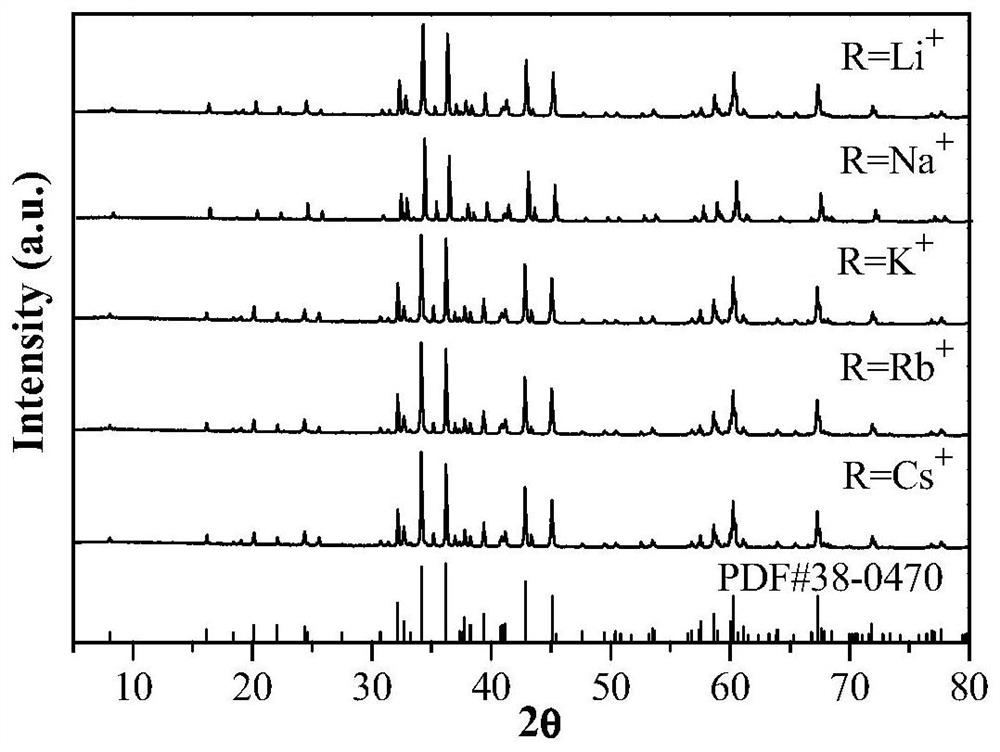
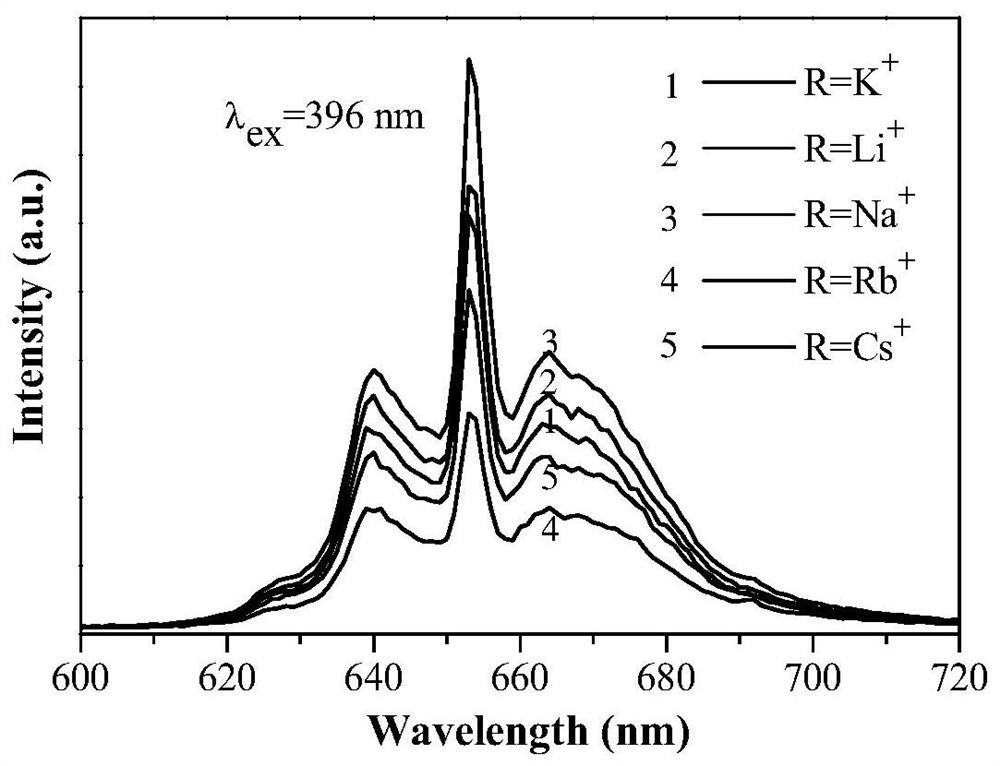
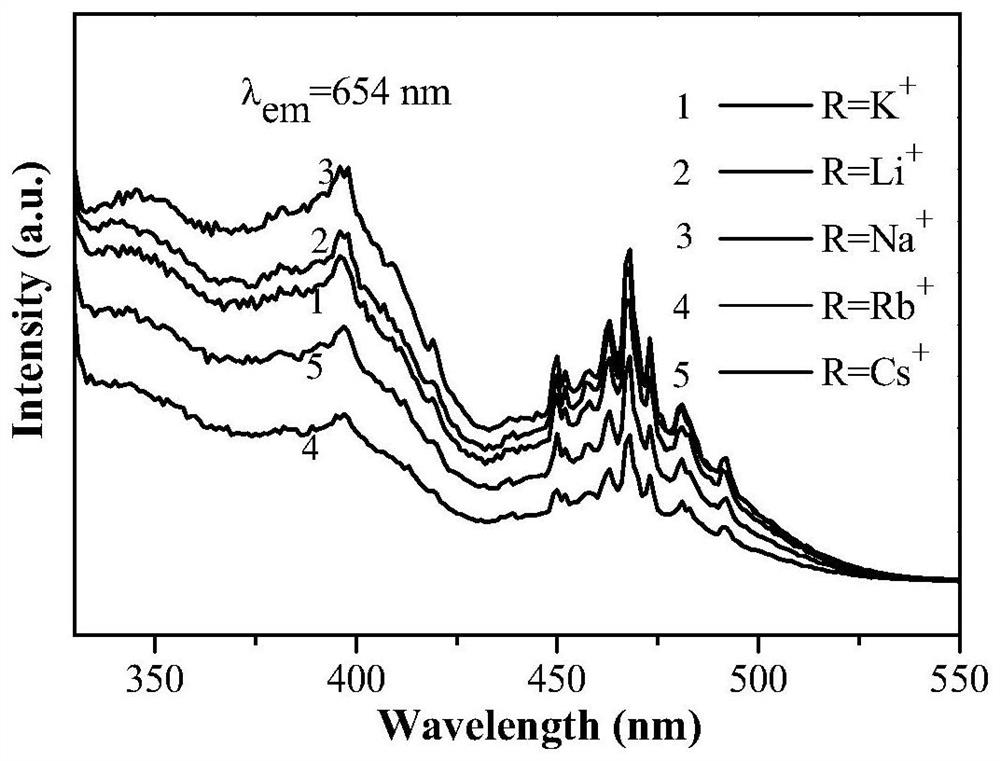
[0047] According to CaAl 11.79 P 0.1 o 19.1 :0.01Mn 4+ ,0.1R + The stoichiometric ratio of Ca, Al, P, Mn, Li in (R=Li), respectively weigh calcium carbonate 0.1000g, manganese carbonate 0.0012g, lithium fluoride 0.0026g, aluminum hydroxide 0.9196g and ammonium dihydrogen phosphate 0.0115g, the raw materials were mixed and placed in an agate mortar, 2ml of absolute ethanol was added and ground, and the obtained mixture was placed in a muffle furnace for calcination under an air atmosphere at a calcination temperature of 1200°C and a calcination time of 2h. After the calcination, it was naturally cooled to room temperature, the sample was taken out and ground, and sealed for future use to obtain a Li + Ionic charge compensation type Mn 4+ Activated aluminophosphate luminescent materials.
Embodiment 2
[0049] According to CaAl 11.79 P 0.1 o 19.1 :0.01Mn 4+ ,0.1R + The stoichiometric ratio of Ca, Al, P, Mn, Na in (R=Na), weigh calcium carbonate 0.1000g, manganese carbonate 0.0012g, sodium fluoride 0.0042g, aluminum hydroxide 0.9196g and ammonium dihydrogen phosphate 0.0115g. Mix the weighed medicines in an agate mortar, add 2ml of absolute ethanol and grind them. The obtained mixture is calcined in an air atmosphere in a muffle furnace. The calcining temperature is 1200° C. and the calcining time is 2 hours. After the calcination, it is naturally cooled to room temperature and ground evenly to obtain a Na + Ionic charge compensation type Mn 4+ Activated aluminophosphate luminescent materials.
Embodiment 3
[0051] According to CaAl 11.79 P 0.1 o 19.1 :0.01Mn 4+ ,0.1R + The stoichiometric ratio of Ca, Al, P, Mn, K in (R=K), respectively weigh calcium carbonate 0.1000g, manganese carbonate 0.0012g, potassium fluoride 0.0581g, aluminum hydroxide 0.9196g and ammonium dihydrogen phosphate 0.0115g. Mix the weighed medicines in an agate mortar, add 3ml of absolute ethanol and grind, and place the obtained mixture in a muffle furnace for calcination under an air atmosphere at a calcination temperature of 1200°C and a calcination time of 2 hours. After the calcination, it is naturally cooled to room temperature and ground evenly to obtain a K + Ionic charge compensation type Mn 4+ Activated aluminophosphate luminescent materials.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com