Preparation method of alpha-substituted-beta-hydroxynitrile compound and derivative thereof
A technology of compound and hydroxynitrile, which is applied in the field of preparation of α-substituted-β-hydroxynitrile compounds and their derivatives, can solve unsatisfactory results of enantioselectivity and diastereomer selectivity, stereochemical results Difficult to control, low reaction yield and other problems, to achieve the effect of excellent diastereoselectivity and enantioselectivity, mild reaction conditions, and less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
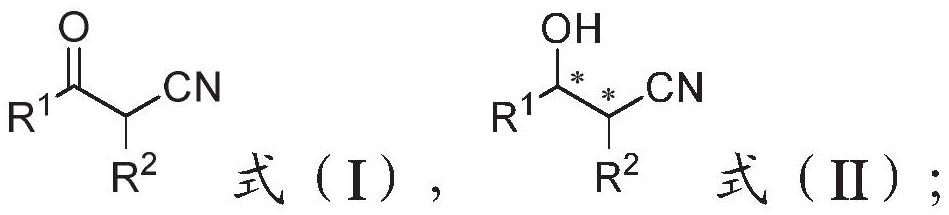
[0006] The present invention provides a kind of preparation method of α-substituted-β-hydroxy nitrile compound, comprising:
[0007] The compound of formula (I) structure is reacted under metal catalyst effect, obtains the compound of formula (II) structure,
[0008]
[0009] Among them, R 1 C6-C30 unsubstituted aryl, C6-C30 substituted aryl, C4-C20 unsubstituted heteroaryl, C4-C20 substituted heteroaryl, C1-C10 alkyl or C3-C10 cycloalkane base;
[0010] R 2 It is an unsubstituted aryl group of C6-C30, a substituted aryl group of C6-C30, an alkyl group of C1-C10 without substituents or an alkyl group of C1-C10 containing substituents;
[0011] or R 1 , R 2 Together with the carbons where they are located, they form a cycloalkyl group or a cycloalkyl-containing parallel ring or condensed ring structure;
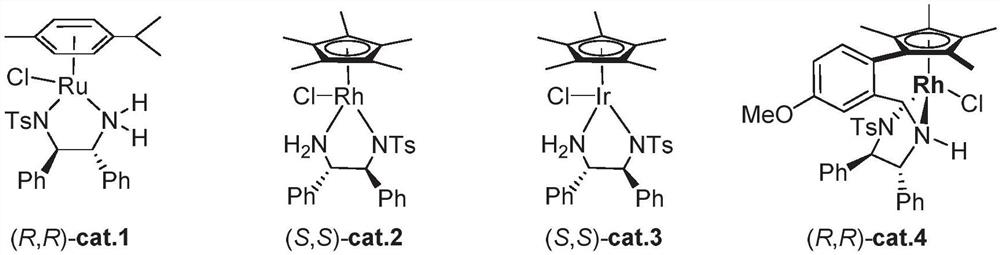
[0012] The metal catalyst is one or more of ruthenium catalyst, iridium catalyst and rhodium catalyst.
[0013] According to the present invention, the present inven...
Embodiment 1
[0043]
[0044] Under the argon atmosphere in the glove box, the metal catalyst cat.4 (0.002mmol), the compound represented by formula I (0.2mmol), formic acid / triethylamine (5:2), and toluene were respectively added to a 10mL Shrek bottle , the system was stirred at room temperature for 12 h. After the reaction is completed, NaHCO3 solution is added to the system to quench the reaction, and the organic phase obtained by extracting twice with EA is dried and concentrated, and flash column chromatography can obtain the pure compound shown in formula II. Apply HNMR to determine the purity and yield of the product, and use HPLC to detect the enantioselectivity and diastereoselectivity of the reaction.
[0045] specific:
[0046] Under the argon atmosphere in the glove box, add different metal catalysts cat.1~cat.4 (0.002mmol), the compound represented by formula 1a (0.2mmol) and formic acid / triethylamine into four 10mL Shrek bottles respectively (5:2) azeotrope (50uL) and di...
Embodiment 2
[0055] Under an argon atmosphere in a glove box, add the metal catalyst cat.4 (0.002 mmol), the compound represented by formula 1a (0.2 mmol) and formic acid / triethylamine (5:2) azeotrope into a 10 mL Shrek bottle (50uL) and organic solvent (2mL), stirred at room temperature for 12h, and detected the reaction;
[0056] According to the above preparation method, only the type of organic solvent is changed, the reaction is carried out, and the reaction situation is detected.
[0057] The obtained results are shown in Table 2.
[0058] Table 2: Effect of different organic solvents on the stereoselectivity of products
[0059]
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com