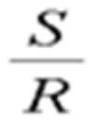Method for manufacturing ruthenium oxide-supported catalyst for preparing chlorine and catalyst manufactured thereby
A technology of supported catalyst and ruthenium oxide, which is applied in the direction of catalyst activation/preparation, preparation with chloride, metal/metal oxide/metal hydroxide catalyst, etc., can solve the composition or effect of loaded ruthenium components, and does not specifically provide And other problems, to achieve the effect of expanding scale, high conversion rate of hydrogen chloride, high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] According to an embodiment of the present invention, a preparation method of a ruthenium oxide supported catalyst is provided, the ruthenium oxide supported catalyst is a ruthenium oxide catalyst for chlorine production, comprising the following steps: step (a), dissolving the ruthenium compound in an organic solvent to prepare a solution, and loaded on more than one carrier selected from titanium dioxide and alumina; step (b), drying; step (c), firing.
[0040] Different from the known technology, the catalyst of the present invention does not need alkali pretreatment in order to only contain the ruthenium oxide component in the shell layer on the outer surface of the titania carrier. Therefore, it can be easily prepared in three steps of loading, drying, and firing. Therefore, the preparation method can be simplified while maintaining the catalytic activity, which is beneficial for future scale-up. This can provide favorable effects in terms of time and economy of th...
Embodiment 1
[0077]
[0078] 40.0 g of titanium dioxide powder, 0.8 g of an organic binder, 29.0 g of ultrapure water heated to 60° C., and 5.0 g of titanium dioxide sol were mixed. The obtained mixture was extruded into a noodle-like strand with a diameter of 2.0 mmφ, dried in air at 60° C. for 2 hours, and then cut into a molded body with a length of 2 to 4 mm. The obtained compact was fired in air at 600° C. for 3 hours.
[0079]
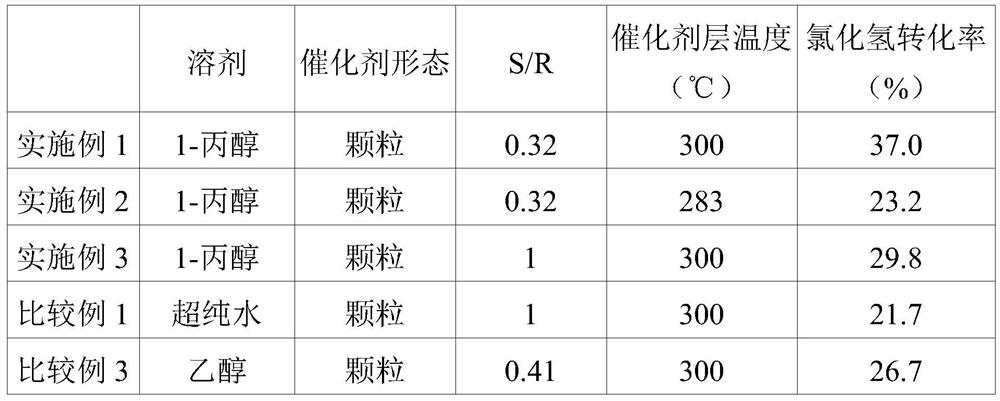
[0080]After impregnating 5.0 g of the titania support obtained above with a solution prepared by dissolving 0.2 g of ruthenium chloride hydrate in 1.33 g of 1-propanol, drying was performed in air at 100° C. for 4 hours. The dried solid was calcined at 350° C. for 3 hours in an air-flowing electric furnace, and then slowly cooled to room temperature to finally obtain a ruthenium oxide catalyst with a ruthenium oxide content of 2.0 parts by weight. In addition, the degree of outer surface loading of the ruthenium component measured by VMS was 0.32.
[...
Embodiment 2
[0086] For the catalyst obtained in Example 1, the initial activity evaluation was performed in the same manner as in Example 1 except that the temperature of the catalyst layer used was 283°C. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com